EVs in tumor development
Extracellular vesicles (EVs), including exosomes, play a crucial role in tumor biology. Alongside modulating the tumor microenvironment and contributing to the malignant cascade (angiogenesis, the host immune system response, and metastasis), EVs are involved in chemoresistance. Docetaxel in prostate cancer, for example, may transport mRNA encoding drug-resistant proteins or carry miRNA to recipient cells and trigger the expression of specific target genes.
These functions make them an excellent target for the development of novel types of cancer treatment, including:
- Biomarkers in diagnostic and characterization of patient-specific tumors
- Targets for anti-cancer therapies to regulate intercellular communications and affect the immune response, apoptosis, vascularization, proliferation and migration of cancer cells
- Cargos for drug delivery, as EVs can carry small molecules, siRNA, peptides, antigens and other biologics
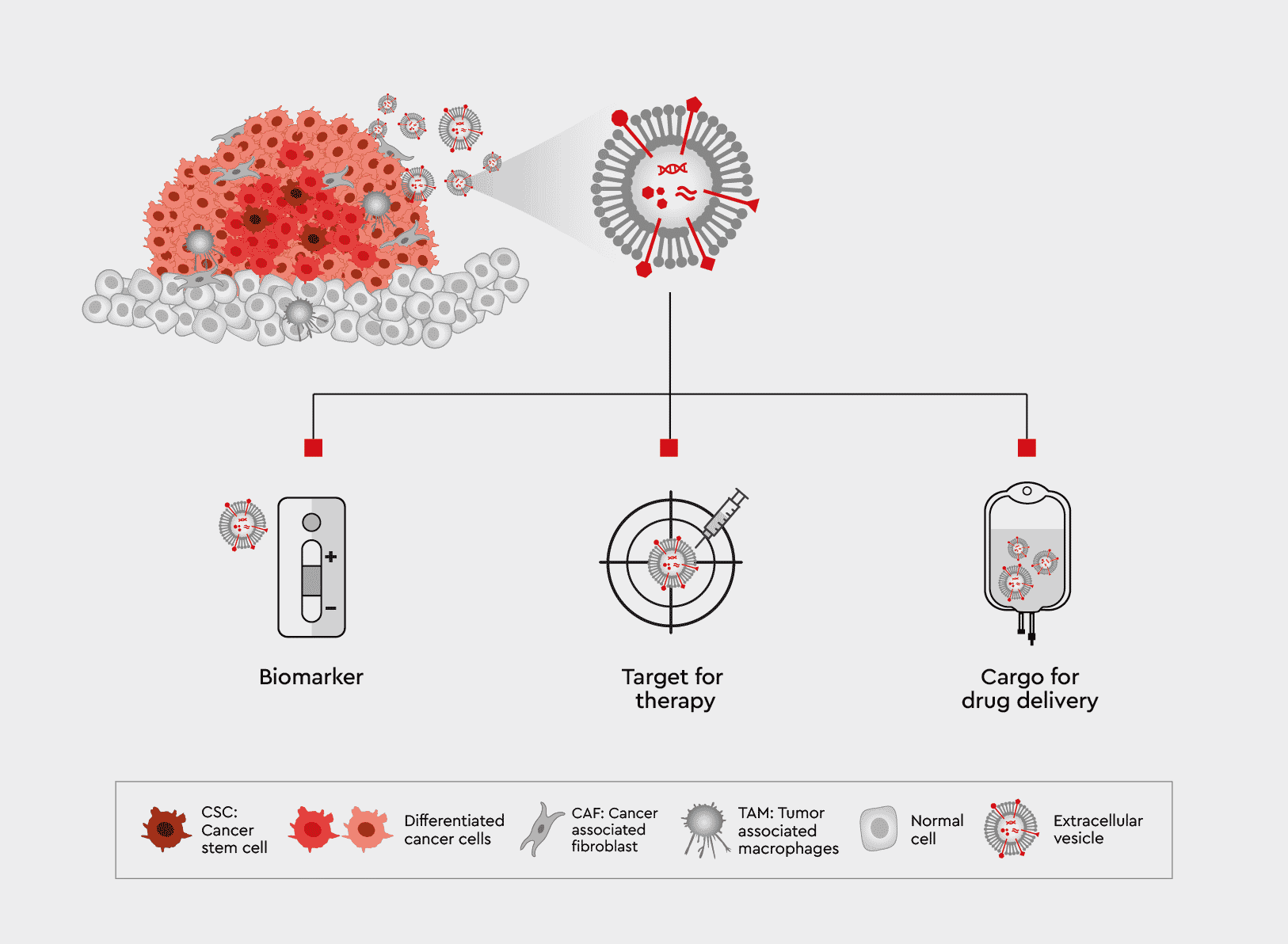
Figure 1: The role of EVs in tumor biology and possibilities of treatment for cancer.
Despite their essential role in tumor biology and promise as therapeutic agents, the identification and production of EVs offer different challenges that have yet to be overcome:
- Access to cell culture media providing sufficient cell growth of cancer cells and production of pure EVs
- Obtaining EV from patient-derived tumor samples
- Characterization of EVs and drug-loaded EVs to assess efficacy and safety in tumor models
- Cell Culture media for the large-scale EV production under GMP conditions
Cancer cell-derived EVs
First vesicle-free growth media to harvest EVs from cancer cell lines
As EVs are secreted from cancer cells, the endosomal/cellular process of EV production depends on cell growth. Thereby, it is vital to support the cell growth without changing cell behaviors and characteristics to optimize the exosome yield.
The problem already starts when choosing an appropriate medium that is, on the one hand, free of EVs and, on the other hand, sufficiently supports the cell growth. Serum-free, chemically defined media or media from which the vesicles have been removed often lack sufficient nutrients or cell growth factors to warrant a proper cell culture over several days or weeks. Furthermore, these media can induce cell stress and impair the EV yield.
We have developed a toolbox of serum- and xeno-free media for cancer cultures, which support healthy growth and improved expansion of cells over many weeks. Additionally, the media can be used for cells from all different sources, offering the great benefit that only one medium can be used for different cancer cell cultures.
The advantage for your EV production:
- Only one medium is sufficient to support many different cancer cell lines, independently of the origin
- Improved expansion and growth performance compared to serum-containing media
- The serum- and xeno-free formulation is vesicle-free
- GMP-compliant manufacturing is possible
- Available for large-scale production in media bags
The Cancer Cell Line Medium XF is a vesicle-free medium (< 1% compared to FCS containing conventional media). As part of our cancer media toolbox, it is a synthetic medium, but in contrast to many chemical-defined media or vesicle-depleted media, it is a fully-fledged growth medium to enable long-term cultivation and multiple passaging.
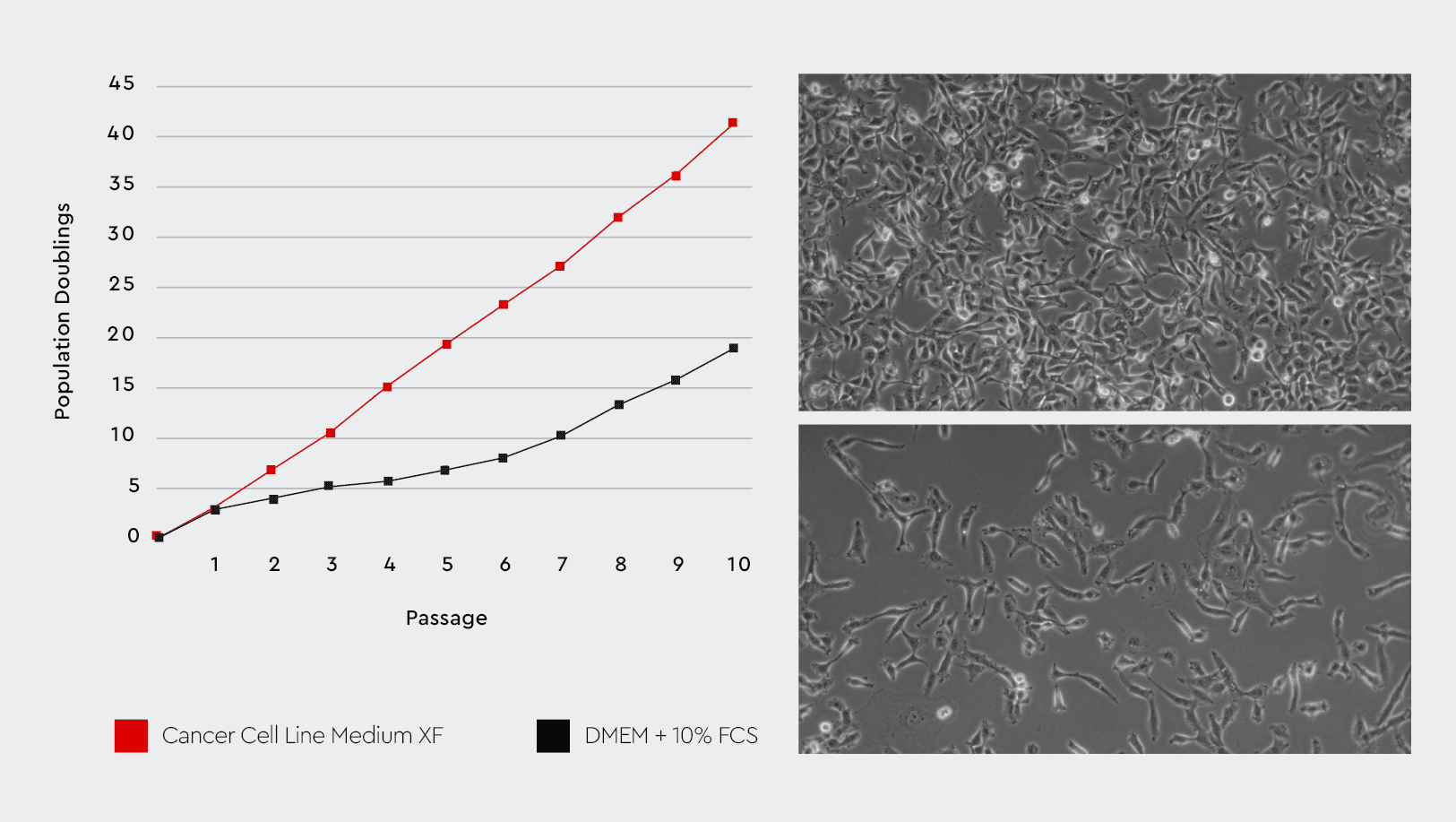
Figure 2: The Cancer Cell Line Medium XF provides better cell growth performance compared to conventional serum-containing media. Left: The Cancer Cell Line Medium XF improves the growth performance of cancer cells compared to a conventional FCS-substituted medium. Right: The cell characteristics and morphology are maintained in the Cancer Cell Line Medium XF (upper right) compared to the serum-containing medium (lower right).
EVs as cancer biomarkers
Efficiently harvest EVs from patient-specific cancer cell cultures
EVs can be isolated directly from the body from a variety of biological fluids including plasma, urine, semen, or tears. However, the complex composition of these fluids, such as metabolites, proteins or macro molecules, disturb the identification and analysis of cancer-specific EVs. Additionally, investigating cancer-derived EVs and identifying new cancer biomarkers requires cancer cell-specific EVs. EVs must subsequently be isolated from pure cancer cell cultures in a vesicle-free environment.
We have developed a cancer media toolbox for the efficient isolation and expansion of patient-derived cancer cells, including tumor-initiating cancer stem cells. These are synthetic media that can be used individually or in combination to select pure CSCs or CSCs together with further differentiated cancer cells while preserving the characteristics of the cancer cells.
Using our cancer media, you can isolate EVs from pure patient-specific cancer cells as a reference to the EV-isolation from liquid biopsies.
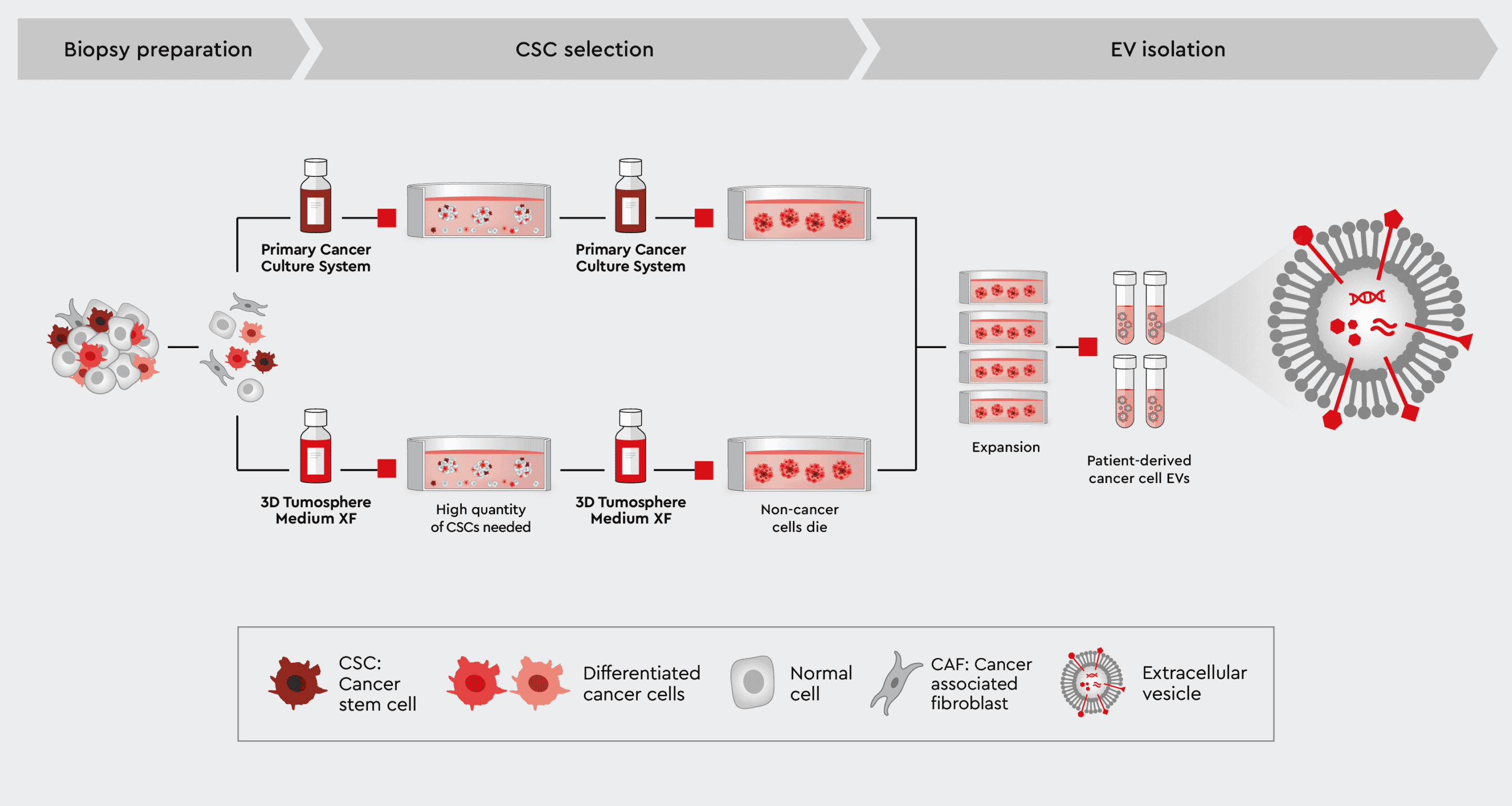
Figure 3: Workflow to harvest EVs directly from patient-specific cancer cells
Patient-derived cancer cells can also serve as a great tool to investigate EV-mediated cell-cell and cell-matrix interactions.
If you want to learn how to establish your own patient-derived 2D and 3D models including CSC, cancer cells and non-cancer cells such as immune cells and fibroblasts you can find an overview on our website From Biopsy to Assay Development.
GMP-compliant EV large-scale production
EVs are increasingly pivotal instruments for drug delivery, drug development and diagnostics. Therefore, the demand for media is increasing, especially for those which can be used in a clinical setting and which are GMP-compliant.
We are a trusted and certified manufacturer of cell culture media compliant to GMP regulatory requirements. This ensures the access to documentation required for your risk assessment to use our innovative media for clinical applications.
- Our Cancer Cell Line Medium XF can be obtained compliant to relevant GMP guidelines
- The medium has a high lot-to-lot consistency and come with comprehensive documentation
- The GMP-medium is fully compatible with the media toolbox to avoid adaptation of cell culture conditions for GMP manufacturing and clinical applications of exosomes
Ask our experts how to set up a full manufacturing process using our GMP-compliant media.
