 The skin is a highly sophisticated organ, yet typically receives little attention. It forms an essential barrier between the body and its environment and is rich in immune cells to combat any opportunistic microbes1,2. In addition, it enables us to interact with our surroundings, e.g., through touch, and also adapt to environmental changes, e.g., temperature regulation3,4.
The skin is a highly sophisticated organ, yet typically receives little attention. It forms an essential barrier between the body and its environment and is rich in immune cells to combat any opportunistic microbes1,2. In addition, it enables us to interact with our surroundings, e.g., through touch, and also adapt to environmental changes, e.g., temperature regulation3,4.
Indeed, the skin is vital for survival, and this is reflected in its capacity to regenerate5. However, in cases where damage to the skin is too great for it to repair itself, such as major trauma/burns/surgical excision or where local skin conditions continually preclude effective healing, e.g., ulceration, medical intervention is required. This may be in the form of negative pressure wound therapy, maggots, growth factors, or bioengineered skin scaffolds6,7,8. Despite the range of available measures for assisted wound healing, chronic wounds remain a significant issue9. As well as not always resulting in successful wound healing, current treatment options for chronic or severe wounds are generally painful and expensive.
Improved understanding of the biological and clinical mechanisms that underpin wound repair is thus needed to inform the development of novel effective treatment strategies for non-healing wounds. Such research requires skin wound models that accurately replicate real skin tissue and in vivo healing processes.
Models for studying wound healing
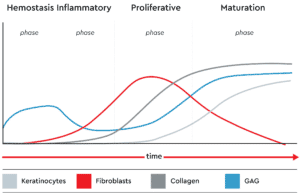
Wound healing requires a complicated cascade of events that begins with hemostasis to stem the bleeding and provide a scaffold for new skin growth. This initiates the inflammatory phase involving neutrophil recruitment and monocyte transformation, leading to the main proliferative healing process, during which granulation tissue forms and the vascular network is restored. Finally, in the maturation phase, local fibroblasts populate the area and re-epithelialization begins.
To understand how best to encourage and support healing, it is necessary to characterize the interactions between different cell types, especially fibroblasts and keratinocytes, that occur during each phase for proper and complete wound closure and healing. To facilitate the research needed to obtain this information and to assess potential novel treatments in an ethical and cost-effective manner requires sophisticated skin models that come close to replicating in vivo conditions.
Challenges to creating wound healing models
The mouse has commonly been used as a model for wound healing, but skin repair mechanisms in the mouse differ considerably to human wound healing processes. Consequently, findings in the mouse do not often translate to benefits in man in clinical studies10. Therefore, there is a need for in vitro models that provide a closer approximation to natural human wound healing.
Wound conditions in animal model systems are often artificial; more relevant models for studying human diseases can be achieved using primary cells isolated from the human body. For example, fibroblasts have been isolated from chronic wounds and used to study the cellular mechanisms at work in non-healing wounds11. Such cell models provide a highly relevant model for investigating chronic wounds, but cannot give the whole picture. Primary cell cultures are typically studied in isolation and so cannot show how the processes in the cell type studied affect other cell types in the surrounding area. In the complex sequence of events that occur during normal wound healing, it is likely that fibroblasts have stimulatory or inhibitory effects on the function of other cell types, for example, stromal cells and keratinocytes.
Despite the limited information obtained from two-dimensional (2D) wound healing models, these are used in most studies due to the challenges of successfully co-culturing a mixture of different cell types. Typically cultures of cells are grown in monolayers and exposed to biological factors known to be effectors of healing or ‘injured’ by scraping to form a ‘wound’ that is subsequently filled with new or migrating cells. Such cell models obviously differ from the physiological cellular environment that has a complex three-dimensional (3D) structure12.
Although some 3D models were developed, e.g., using electrospun fiber membranes to form 3D scaffold for cell cultures, these are often highly laborious and require technical expertise13. Furthermore, existing wound healing models do not take into account the different phases, and treatments may have a different impact depending on the phase at which they are applied14.
There was thus the need for an easy-to-use 3D wound healing model that enables the study of the different phases and cell types involved in the natural wound healing process.
High-throughput 3D in vitro wound healing model
Recently 3D wound healing models capable of replicating distinct phases of the natural wound healing process have been produced using magnetic 3D bioprinting15.

Fibroblasts and keratinocytes are magnetized using biocompatible NanoShuttle™-PL1416,17. One spheroid per well can then be formed using mild magnetic forces that induce cell aggregation. In this way, structurally and biologically representative 3D cellular constructs can be easily created in vitro without the need for a scaffold. By using different ratios of fibroblasts and keratinocytes in different wells, the different phases of wound healing can be evaluated in a single wound healing model. In addition, using a 384-magnetic ring drive enables high throughput screening of the different phases of healing.
Furthermore, the wound healing process can be visualized continuously using live-cell imaging in this 3D wound healing model. CytoSMART® Omni automatically images and analyzes wound closure every hour in each well of the 384-well plate18.
In addition to facilitating research into the development of novel treatment approaches to improve wound healing, the magnetic 3D cell culture methodology will aid other experimental approaches in the field of regenerative medicine and accelerate advances in personalized cancer treatments19,20.
Summary
A readily reproducible, high-throughput 3D in vitro wound healing model has been developed that accurately reflects physiologic healing and is not labor-intensive. It uses Greiner’s magnetic cell culture technique and the CytoSMART live imaging technology. Creating individual spheroid cell constructs in separate wells of a 384-well plate using varying compositions of keratinocytes and fibroblasts allows the different phases to be screened with high throughput.
References
- Grice EA, Segre JA. Nat Rev Microbiol. 2011;9(4):244-253.
- Richmond JM, Harris JE. Cold Spring Harb Perspect Med. 2014;4(12):a015339.
- Slominski AT, et al. Adv Anat Embryol Cell Biol. 2012;212:v-115.
- Romanovsky AA. Acta Physiol (Oxf). 2014;210(3):498-507.
- Takeo M, et al. Cold Spring Harb Perspect Med. 2015 Jan 5;5(1):a023267.
- Huang C, Tet al. Current Problems in Surgery 2014;51(7):301-331.
- Mohd Zubir MZ, et al. Int J Environ Res Public Health. 2020 Aug 21;17(17):6103.
- Nour S, et al. J Biomed Mater Res A. 2021 Apr;109(4):453-478.
- Olsson M, et al. Wound Repair Regen. 2019;27(1):114-125.
- Zomer HD, TRentin AG. Journal of Dermatological Science 2018;90(1):3-12.
- Caley M, et al. Int J Mol Sci. 2018;19(4):1001.
- Grazul-Bilska AT, et al. Drugs Today (Barc) 2003;39(10):787–800.
- Kapałczyńska M, et al. Arch Med Sci. 2018 Jun;14(4):910-919.
- Iyer K, et al. Tissue engineering and regenerative medicine 2018;15(6):721–733.
- PromoCell application note. https://promocell.com/wp-content/uploads/2022/01/3D-wound-healing-app-note
- https://3dcellculture.gbo.com/
- Dias JR, et al. Advances in electrospun skin substitutes. Progress in Materials Science, 84:314–334, 2016.