The uniform characterization of Human Mesenchymal Stem Cells
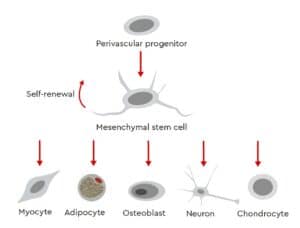
Mesenchymal stem cells (MSCs) are fibroblastoid multipotent adult stem cells with a high capacity for self-renewal (Fig. 1). These cells have been isolated from several human tissues, including bone marrow, adipose tissue, umbilical cord matrix, tendon, lung and periosteum (da Silva Meirelles, et al. 2008 ; Ullah, et al. 2015).
Investigators are using different methods for cell isolation and expansion and take different approaches for characterizing their MSCs. These inconsistent defining characteristics of MSCs make it difficult to determine whether the cells used in studies and experiments are similar enough for the results and outcomes across different groups to be compared and contrasted.
According to recent analyses, billions of dollars are spent each year in the United States alone on preclinical research that is not reproducible (Freedman, et al. 2015). The factors contributing to the lack of reproducibility in preclinical experiments include poor study design, use of different biological reagents and inconsistent reference materials. The reproducibility of basic and preclinical research can be substantially improved by using validated research materials and technologies and adopting the best available practices thus accelerating progress in the field and favoring the subsequent development of clinical therapies.
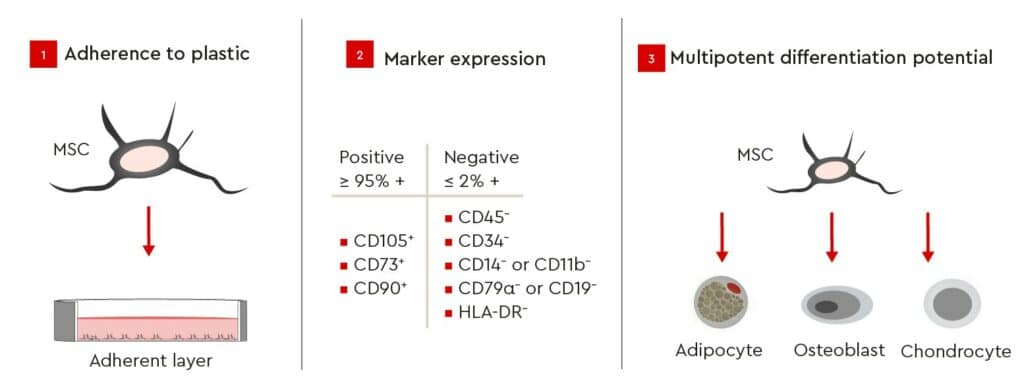
To address these issues, the International Society for Cellular Therapy (ISCT) has defined three minimum criteria (Fig. 2) for ensuring the integrity and unambiguous identification of human MSCs in order to provide a common set of comparable standard criteria for MSC research (Dominici, et al. 2006). According to these guidelines, MSCs must be plastic-adherent when kept under standard culture conditions and express specific surface markers (Tab. 1). In addition, MSCs must be able to differentiate into adipocytes, osteoblasts, and chondrocytes under standard in vitro differentiating conditions (Fig. 2).
Cell Identity
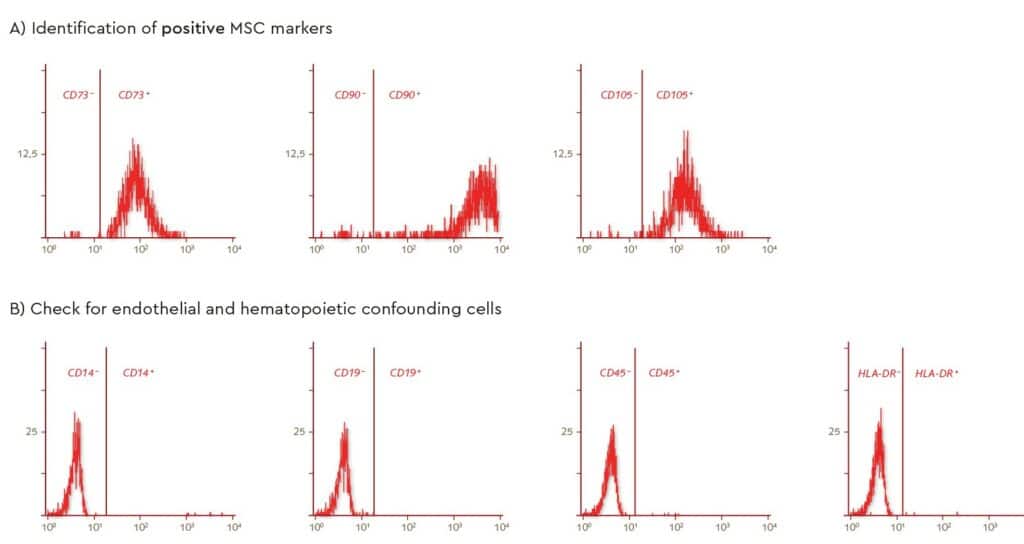
Characterization of MSC surface markers by flow cytometry, following isolation from bone marrow using PromoCell’s MSC Growth Medium, showed a defined MSC population according to the recommended ISCT markers for determining MSC identity (positive markers in Fig. 3A and negative markers Fig. 3B).
Self-renewal
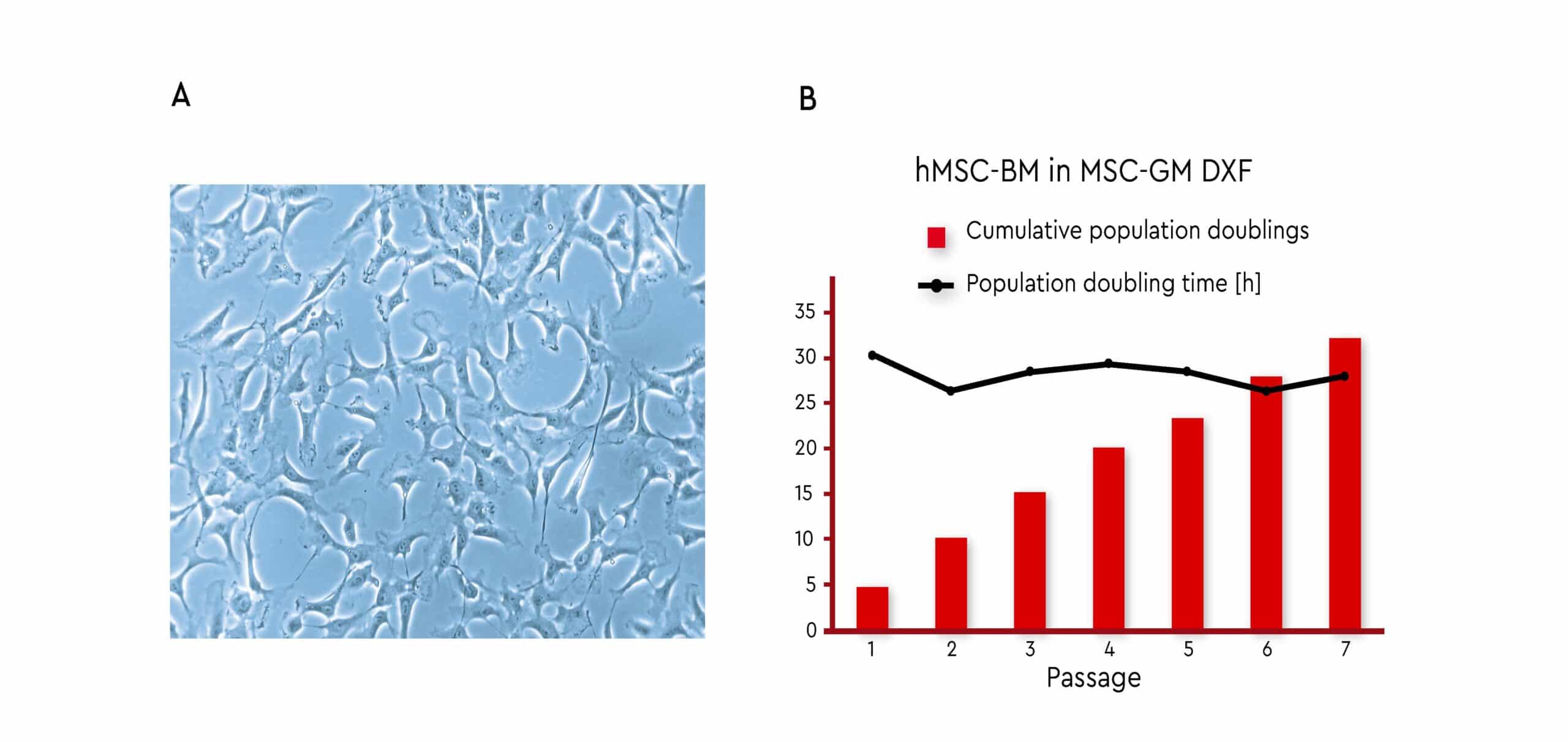
Expansion of bone marrow-derived MSCs using PromoCell MSC Growth Medium DXF resulted in a stable growth performance over several passages (Fig. 4). The MSCs used in this investigation all meet the ISCT criteria (see Fig. 2), thus demonstrating the self-renewal capabilities of these defined cell populations.
Multipotency
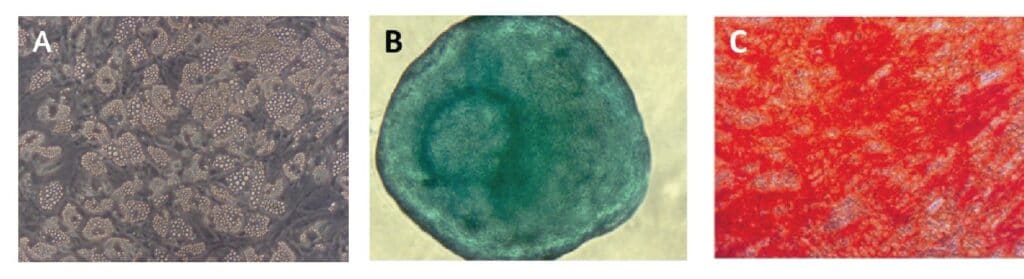
Differentiation of expanded bone marrow MSCs into adipocytes, chondrocytes, and osteoblasts, in accordance with ISCT criteria, was assayed in passage 3 using PromoCell MSC differentiation media (Fig. 5). All of the MSCs tested differentiated successfully into the three cell types, thus demonstrating their multipotency. Adipogenic differentiation exhibited the extensive intracellular lipid vacuole formation typical of mature adipocytes (Fig. 5, A). Chondrogenic differentiation of MSCs was determined by inducing cartilage spheroid formation (Fig. 5, B). Finally, differentiation of MSCs into mature osteoblasts was demonstrated by Alizarin Red S staining of extracellular calcium deposits in mineralized cells (Fig. 5, C).
Conclusions & Future Prospects
Isolation of human MSCs using PromoCell Media yields a uniform, well-defined MSC population in agreement with the marker expression profiles proposed by the ISCT. The stable growth performance and expansion over the course of several passages demonstrate the self-renewing capabilities of these cells. Multipotency has been demonstrated by the differentiation of expanded MSCs into adipogenic, chondrogenic, and osteogenic lineages. Considered as a whole, these data indicate that validation of cellular identity based on the ISCT recommendations gives rise to a well-defined MSC population suitable for in vitro expansion while retaining stemness. The MSC isolation method and growth medium used for culturing play a crucial role in obtaining a well-defined MSC population that is in agreement with the definition included in the ISCT guidelines. Standardizing characterization and culturing of MSCs generates valuable scientific data while ensuring their comparability across different laboratories. It therefore is useful to become the basis for designing experiments in the field. This standardization has the potential to drive both faster progress in preclinical studies and more rapid translation into the subsequent development of clinical applications [4]. What’s more, the use of MSCs from validated sources is increasingly a requirement for obtaining funding for preclinical research projects. The National Institutes of Health (NIH) in the United States, for example, recently tightened its approval process and review criteria to ensure that only the most rigorous, robust and transparent research is funded in order to improve the reproducibility of findings (National Institutes of Health, 2015) .
