Bill Marris, founder and CEO of Google Ventures, says that “Right now is the ‘transistor moment’ for the human body.” Following the 1947 development of the transistor, computing power doubled exponentially. In the same way, Marris believes that healthcare will “improve at the same radical pace” as computing.
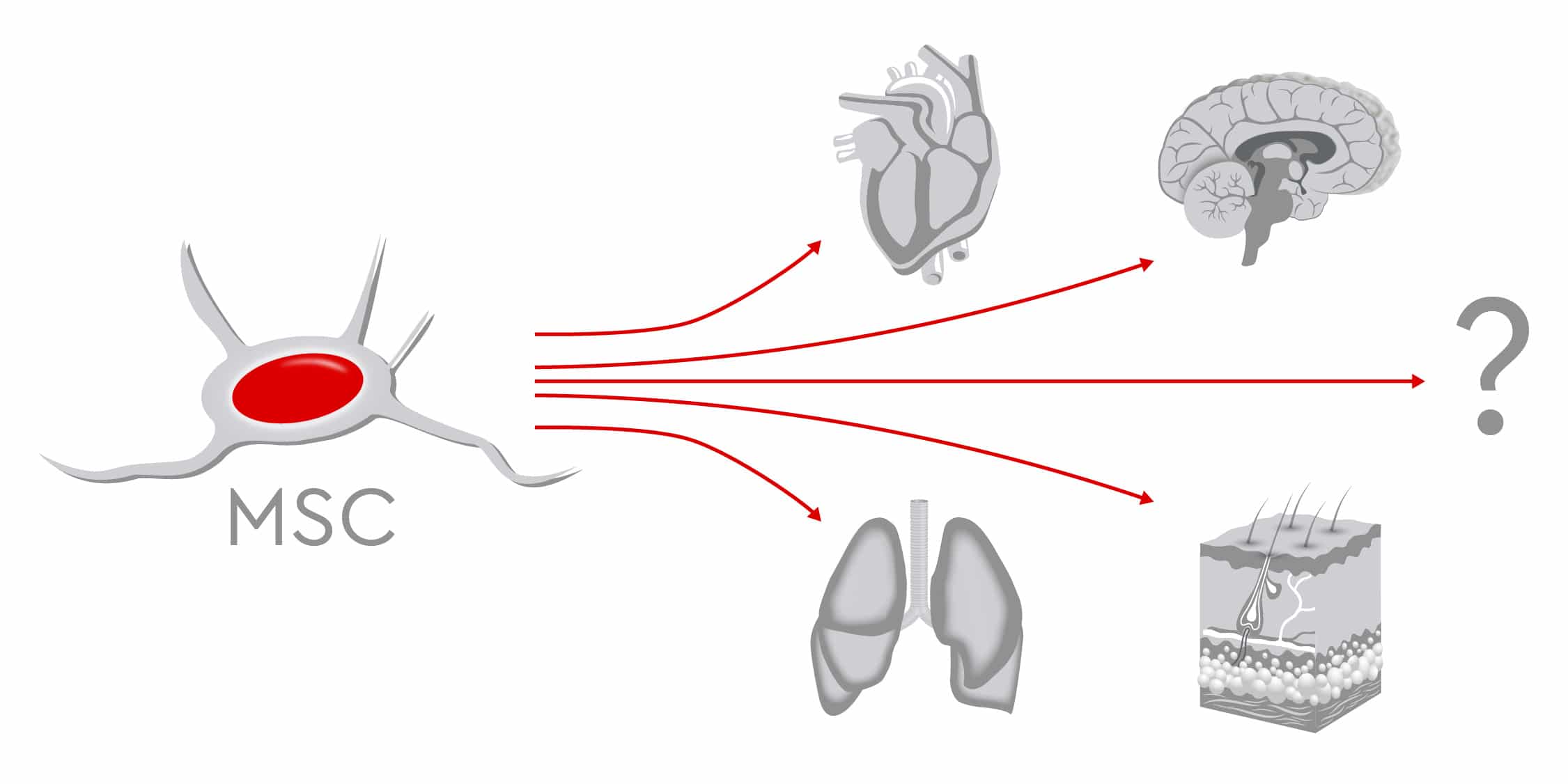
In fact, in some ways, mesenchymal stem cells (MSCs) are not so different from high-tech computer chips. Even the tiniest component must function perfectly for the entire computer to work. Another parallel comes from the exponential growth of computing capacity in recent decades: Production and methods have had to sprint to keep up. Similarly, as the spectrum of potential MSC applications in regenerative medicine continues to increase, manufacturers must now shift to large-scale production. They need to provide a high output of pharmaceutical-grade MSCs made under stringent quality standards. The result: Patients will have sufficient access to these multitalented cells, which can restore normal functioning in the human body.
Stumbling blocks to large-scale MSC production
But at the moment, producing standardized, well-characterized and high-quality MSCs in clinically sufficient quantities is an obstacle course. The first hurdle to be cleared is completing targeted preclinical research. This is mandatory, of course, so that authorities can validate processes and establish quality control criteria. But there is a further challenge: Because of the cellular heterogeneity of MSCs, scientists and industry have difficulty finding common ground. For example, how do they best identify, sort and track cells? And potential stumbling blocks of MSC expansion, freezing and thawing processes, along with final cell characterization, still need to be standardized.
Yet the most troublesome aspect is coaxing these cells into performing optimally. After collection, MSCs need to be expanded in vitro, because cellular therapies require far more cells than what scientist can extract from adult tissue. In fact, MSCs make up just 0.01 percent of mononuclear cells in the bone marrow of adult donors. Their number in bone marrow aspirates is also low. For regenerative medicine to be effective, however, infusions must contain several million cells per kilogram of body weight (Mizukami and Swiech, 2018). This means that the in vitro expansion phase is critical, as it could negatively affect the function of MSCs and therefore their therapeutic potential. Donor-related factors such as age and gender, along with cell culture-related factors, can further influence how well MSCs reproduce (Fossett and Khan, 2012).
Mesenchymal cells are also quite sensitive to their surroundings. When cultured in 2D monolayers, MSCs display signs of senescence. Their proliferation rate decreases, and their morphology changes from spindle shape to a flattened square shape as the number of passages increases (Hoch and Leach, 2014). Too much handling increases the risk of microbial contamination. And researchers do not yet agree on the optimal cell density for growth, with different studies suggesting different plating densities. (Sotiropoulou et al., 2006). Generally speaking, using lower seeding densities seem to support rapid expansion of cells. This makes cell culture procedure less time consuming and more cost-effective. At the same time, cell cultures are less likely to become contaminated or lose characteristic functions (Fossett and Khan, 2012).
Exploring various media, methods and processes
Careful handling and alternative culture systems might motivate cell growth. With the aim to preserve key functional characteristics of MSCs and exploit their full therapeutic potential, researchers are testing various approaches:
- First, using 3D culture systems instead of classical monolayers can help circumvent some limitations of 2D culture. A 3D system better resembles the in vivo situation and the physiological environment, both of which positively affect cellular functions (Egger et al, 2019).
- Second, using xeno-free standardized media can avoid xenogeneic responses. These include uncontrolled influences on MSC differentiation, caused by animal-derived culture supplements that are not well characterized. Regulators advise using xeno-free alternatives when defining safer and standardized protocols for producing therapeutic-grade cellular products (Cimino et al, 2017).
- Third, using bioreactors enables cell expansion in a fully closed, controllable and scalable culture system. This is crucial for high-scale production of MSCs in accordance to good manufacturing practices (GMP) and quality standards. Yet questions remain on using bioreactor systems – such as how to determine the best harvesting method, how to concentrate large volumes of cell solutions quickly without impairing cell viability and function, and when to replace traditional trypsin with newer enzymes that gently collect MSCs from microcarriers (Mizukami et Swiech, 2018).
- Finally, establishing new cryopreservation protocols for the use of nontoxic xeno-free supplements to store MSCs long term. Cryopreservation is the only method that can guarantee cellular products will be available quickly as well as in sufficient quantity to establish clinical-grade MSC banks for therapeutic applications (Rogulska et al., 2019, Lechanteur et al., 2016).
From bench to bedside: why a consistent protocol is key for MSC cell therapy
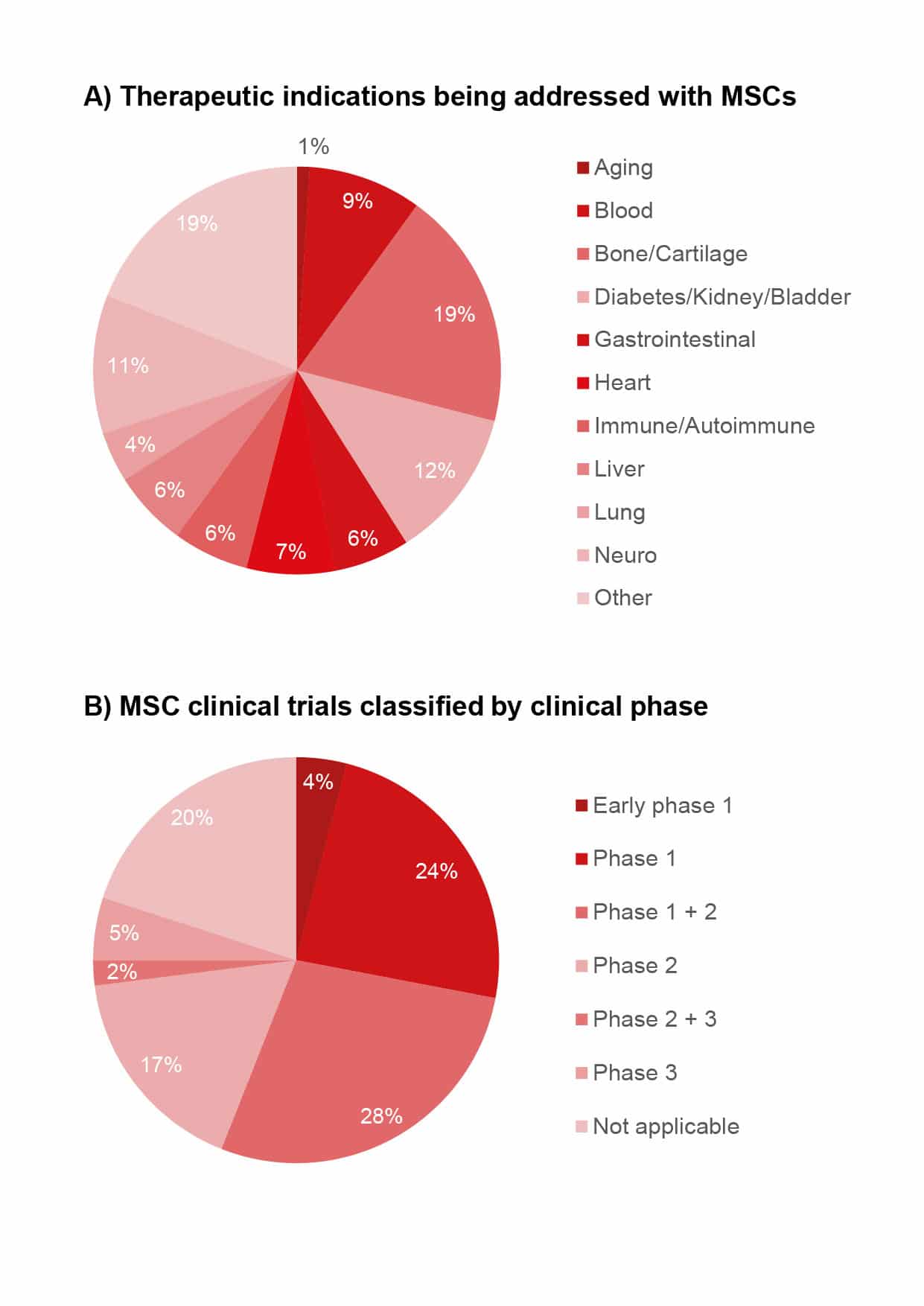
Every new therapy needs a set of ground rules and boundaries. This is why many ongoing preclinical research projects aim to establish regulatory guidelines. Such guidelines ensure that manufacturers produce MSCs according to standardized protocols, since medical experts need to have consistently high-quality cellular products to achieve positive treatment outcomes. In addition, when MSC therapeutics are reasonably priced, they will be used more often. Thus, more efficient manufacturing processes would lead to less expensive products, and lower costs would increase the availability of cell-based therapies for larger number of patients (Olsen et al., 2018). This cost-efficiency is key to unlocking access to the unique features of MSCs and to exploiting their therapeutic potential. As of July 2020, more than 700 recruiting, active or completed clinical studies in the US (clinicaltrials.gov) were investigating human MSCs in areas including heart conditions, neurological diseases, metabolic disorders and autoimmune diseases.
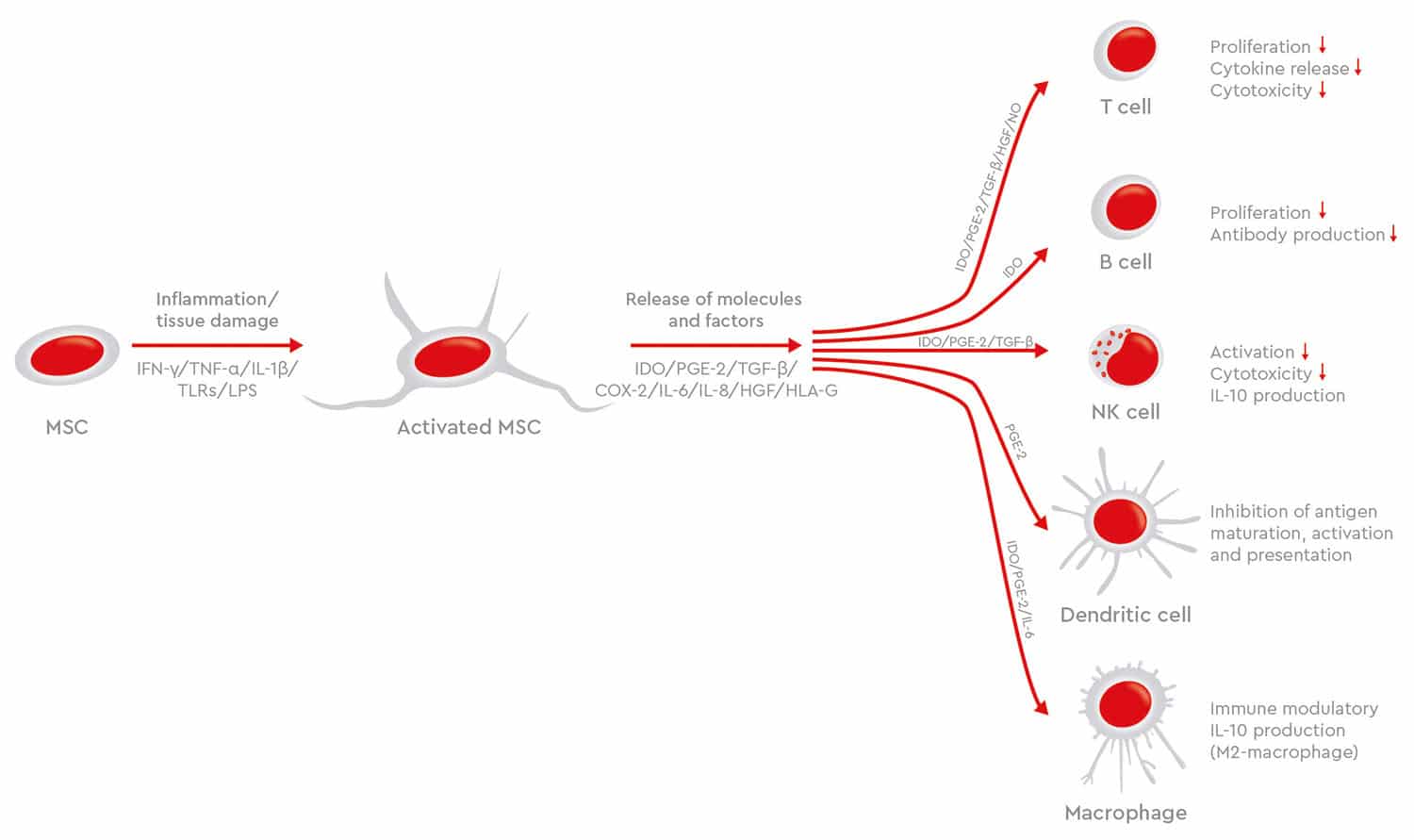
Human MSCs are also showing great promise in the treatment of graft-versus-host disease (GvHD), a common complication in patients receiving allogeneic hematopoietic stem cell transplants. In GvHD, donor immune cells attack recipient’s tissues, such as in the skin, liver and gastrointestinal tract, where they can cause significant damage. Treatment is usually with systemic corticosteroids and immunosuppressants, but the condition often has a poor prognosis. However, the potential of MSCs to modulate the immune system benefit patients with GvHD (Zhao et al., 2019). Though most studies have shown that MSC therapy can improve overall survival for chronic GvHD, the results are sometimes conflicting. The evidence is not robust because of the small number of studies and the limited number of patients treated. Differences in protocols, means of MSC isolation, characterization and manipulation also make it difficult to compare MSC-based treatments. This example shows how critical it is that production protocols are consistent and standardized to deliver better clinical results (Godoy et al., 2019).
Ensuring quality, safety and efficacy
As with all pharmaceuticals, MSC-based therapies must be manufactured according to good manufacturing practices (GMP). However, there is no international consensus regarding quality control standards. At the national level, each research center agrees on production and application guidelines with the local authorities. A recent survey of US facilities that produce MSCs under GMP conditions offers an overview of the current best practices and shows how such practices affect product quality features such as cell yield, cell viability, purity, sterility, expression of surface markers and potency. This survey indicated that most facilities use bone marrow as MSC source, rely on plastic adherence to enrich those cells and use human platelet lysate as a media supplement. However, the study also highlighted a high variability across facilities in practices such as cell-plating densities, harvest times of the cellular product and methods used to assess product identity and potency (Phinney et al., 2019).Most production sites asses quality, safety and efficacy of MSCs based on their identity, morphology, growth characteristics, sterility, karyotype and efficacy in vitro and in vivo. For the International Society Cell and Gene Therapy (ISCT), the three criteria for assessing MSC identity are plastic adherence, phenotypic expression of defined antigens and trilineage differentiation (Dominici et al., 2006). With regard to cellular function, regulatory authorities in both the US and EU require a release assay related to the expected effect of the MSCs product in vivo. But due to the diverse therapeutic applications of MSCs, there is not just one specific test required to prove their efficacy. Nonetheless, the cell therapy community could benefit from a standard set of criteria allowing them to compare the results of clinical studies (Wilson et al., 2019).
The road to cell therapy remains long. Although numerous studies have shown that the clinical use of human MSCs is safe, feasible and effective for specific cases and conditions, regulatory agencies have approved only a few commercial products. The first commercial allogeneic product was approved in 2011 in Korea for treating traumatic and degenerative osteoarthritis. Since then, supported by basic research, other products have been approved for various indications (Mizukami and Swiech, 2018). To speed up the approval process, developers need to resolve scientific issues before they apply for market authorization. This would grant patients faster access to the benefits of new cellular therapies (McBlane et al., 2018).
In the coming decade, the race for commercial MSC-based treatments will certainly accelerate. To match this increasingly rapid pace, the industry will have to quickly establish consistent processes and standardize production. Basic research will be essential to help the industry overcome today’s hurdles and clear the path for a successful clinical application.