What are extracellular vesicles?
EVs are produced by budding cell membranes and released into the extracellular space for intercellular communication. These phospholipid bilayer membrane-coated vesicles carry specific, bioactive proteins and nucleic acids that alter their target cell’s behavior. EVs are mostly classified by their size and biogenesis, with a diameter of 50 nm to 1 µm. There are three types of EVs:1
- Exosomes
- Microvesicles (ectosomes/shedding vesicles)
- Apoptotic bodies
Exosomes are secreted by a variety of different cells, such as stem cells, immune cells, stroma cells, and even cancer cells. These important components of inter-cellular communication are fundamental for numerous physiological and pathological processes.2,8
Role of EVs in cancer biology
Research into EV cancer treatment has become incredibly important in oncology. At the start of the last decade, EVs were shown to play complex roles in the initiation, invasion, metastasis, and recurrence of cancerous cells. They facilitate communication within the tumor microenvironment (TME), transferring bioactive molecules that alter the immune response and tissue remodeling.3
Cancer-derived EVs inhibit the ability of the immune system to identify and destroy tumor cells. EVs help support nutrient transferal to tumors and permit metastasis by promoting angiogenesis within the tumor. Cancer-derived EVs also promote tumor resistance to treatments through the transfer of bioactive molecules that enhance both survival and resistance mechanisms within the tumor.4
The TME composition and the extracellular matrix can be transformed by cancer-derived EVs to encourage tumor growth and invasion. EVs also impact the metabolism of cancerous and stromal cells through the delivery of metabolites and metabolic enzymes to support cancer cells’ energy requirements.4
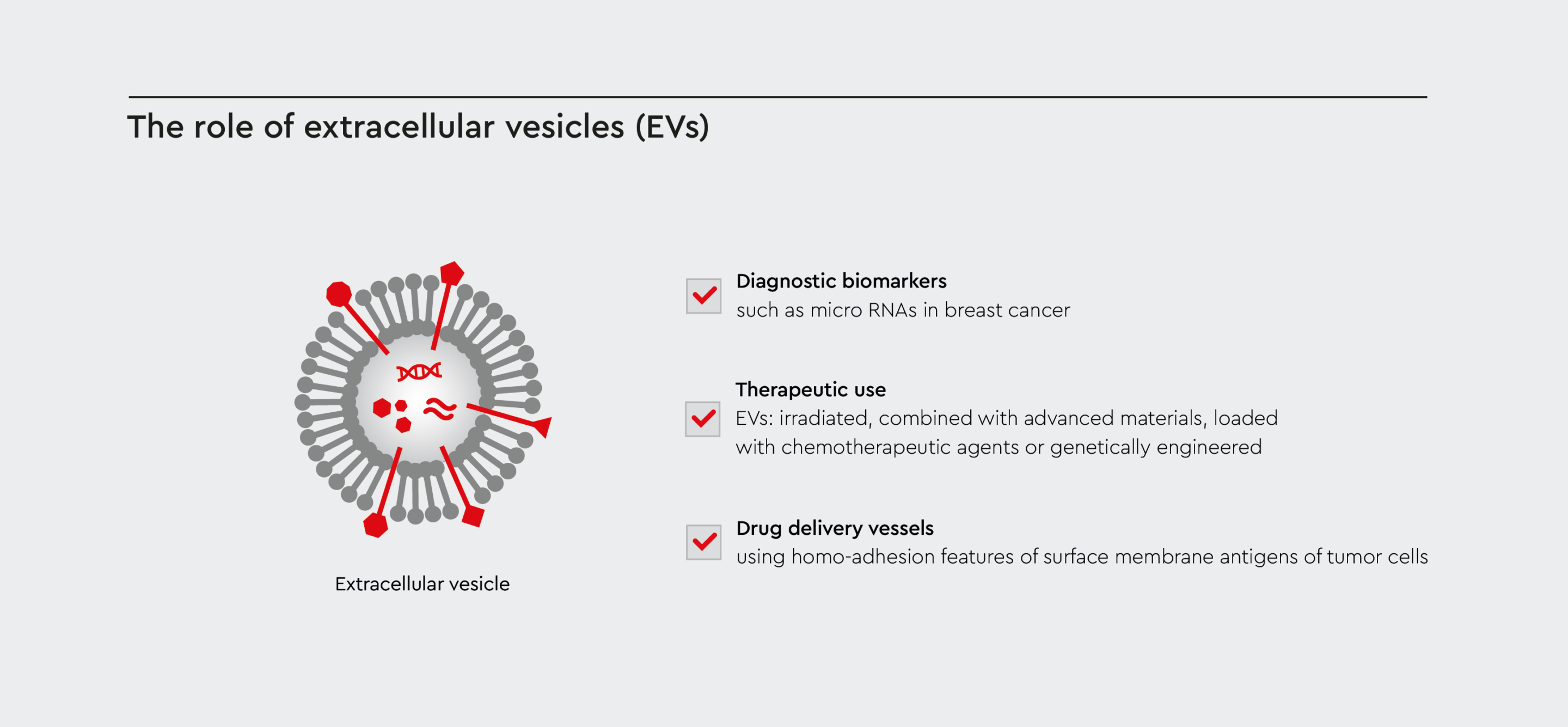
EV cancer treatment and therapy
Research into extracellular vesicles in cancer therapy has attracted a lot of interest recently. As EVs transport molecules that influence recipient cells, scientists are investigating their potential use as:
- Diagnostic markers
- Drug delivery vessels
- Therapeutic agents
Extracellular vesicles as biomarkers
EVs hold huge potential as biomarkers in cancer diagnosis and prognosis because the molecules they transport can mirror the genetic and molecular properties of malignant cells. Exosomal microRNAs have proven to be particularly powerful biomarkers in breast cancer diagnostics. They have revealed different genetic characteristics of different types of breast cancer. Exosomes released by breast cancer cells can also suppress T-cell proliferation and inhibit the cytotoxicity of NK cells, thus reducing the immune response in pre-metastatic tissues.4
Extracellular vesicles for drug delivery
Cancer-derived EVs exploit the intrinsic homo-adhesion features of surface membrane antigens to specifically target tumor cells. This is a promising feature for autologous cancer-derived EVs as drug delivery vessels.5 However, the biology and functions of EVs are not properly understood, and EV cancer treatment is still in its infancy. Therefore, accurate interpretation of their formation, secretion, and interactions is drastically needed to aid the translation of EV cancer treatment into the clinical realm.
Preparing cancer-derived EVs for therapeutic use
Currently, four types of cancer-derived EVs have been classified as potential anti-tumor vesicles in EV cancer treatment research:
- Irradiated cancer-derived EVs
- Cancer-derived EVs combined with advanced materials, such as nanoparticles
- Cancer-derived EVs loaded with chemotherapeutic drugs
- Genetically engineered cancer-derived EVs
Here, they are also working as immune-active agents on top of being manipulated vehicles for anti-tumor drugs. However, problems have arisen in terms of the preparation, efficacy, and applicability of these techniques.1
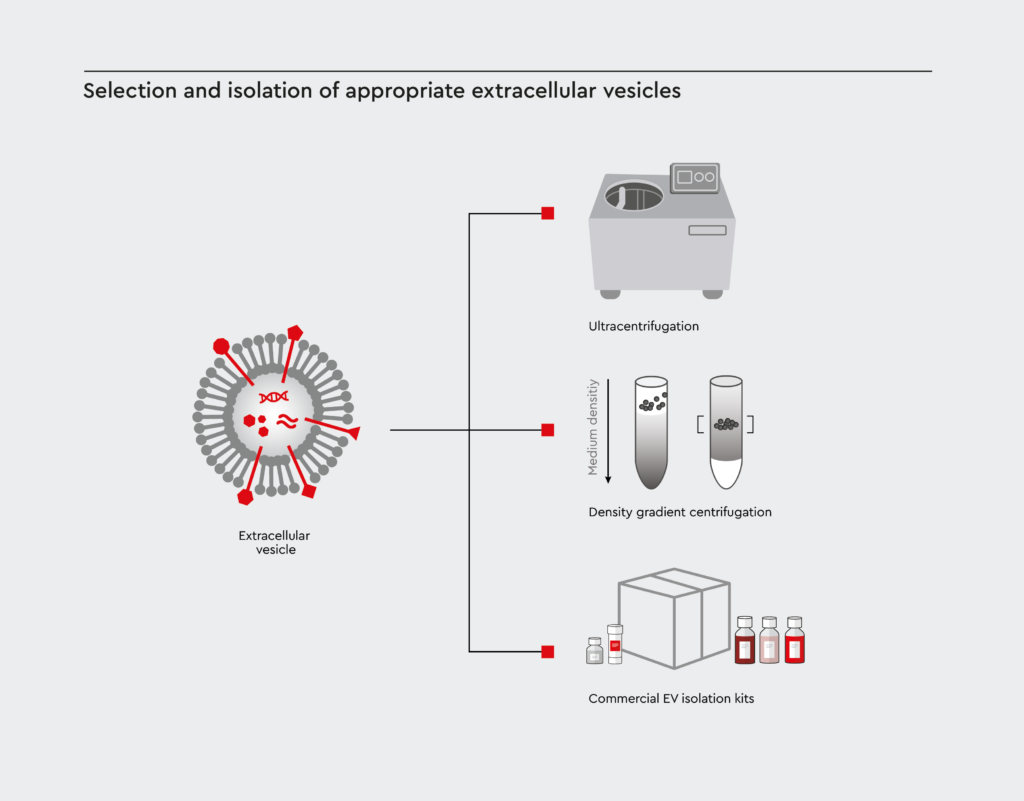
Selection and isolation of appropriate extracellular vesicles
First, the correct cell type involved in the cancer of interest must be selected to produce EVs with relevant biomolecules. Once identified, appropriate cancer-derived EVs must be isolated and purified using methods such as ultracentrifugation, density gradient centrifugation, or EV separation kits.
Quantification and characterization of extracellular vesicles
Isolated cancer-derived EVs that have maintained their integrity and biological cargo must then be quantified and characterized. The type, concentration and size distribution of EVs, along with their cargo, can be confirmed using:
- Nanoparticle tracking analysis
- Electron microscopy
- Proteomic analysis
Efficacy tests
To study the biological functions of cancer-derived EVs in intercellular communication, manipulation of the TME and tumor progression cell cultures is required. Successful experimentation relies on the use of an appropriate cell culture medium that meets certain cell culture requirements. This is necessary to maintain the viability of the EVs. It also ensures that EVs have no contaminants, toxic components or immunogenic molecules that could negatively affect recipient cells.1
With the appropriate cell culture medium, functional assays can effectively test the therapeutic impact of the isolated and adapted EVs on target cells. To test the efficacy of EV cancer treatments, researchers focus on the ability of cancer-derived EVs to:
- Modulate pathways of interest
- Induce apoptosis in targeted cancerous cells
- Affect the overall immune response
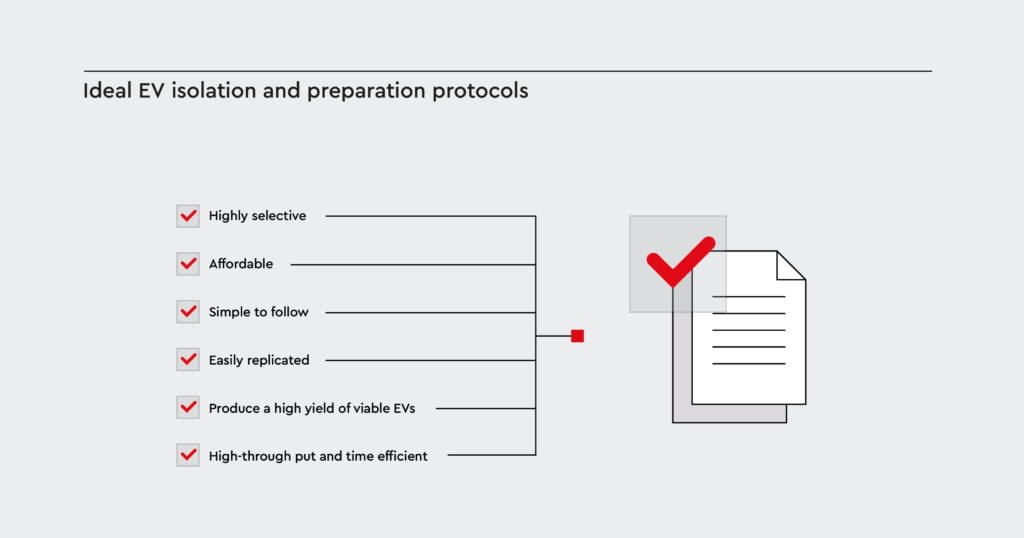
Ideal EV isolation and preparation protocols
Ideal protocols for the isolation and efficacy assessment of cancer-derived EVs must be:
- Highly selective
- Simple to follow
- Affordable
- Easily replicated
- Produce a high yield of viable EVs
- High-through put and time efficient
Strict, replicable protocols are also required to ensure the correct handling and storage of EVs. Appropriate cell culture media are necessary for the maintenance of EV stability and functionality over time.
How we can support your EV cancer treatment research
Our team of experts here at PromoCell provides a cancer media portfolio that offers a practical path for the efficient isolation of pure EVs from patient-derived cancer cells or cancer cell lines, including:
These media are greatly advantageous for EV production because different cancer cell types are supported, and their expansion and growth performance are improved. The cancer media are also available for large-scale EV cancer treatment research.

Not sure which of our Cancer Media Toolbox options is right for you?
Contact our dedicated scientific support specialist for our Cancer Media Toolbox, Dr. Alexander Trampe.
Together, you can explore your intended use and other potential applications, and he can provide you with some tips and information on how to make the most of this medium.
Challenges in isolating cancer-derived EVs
Cell culture-conditioned media provide a more controlled environment for isolated EVs. Several fundamental aspects that can affect EV yields must be considered in EV cancer treatment.
Firstly, it is important to consider whether a culture medium with or without an EV-depleted fetal bovine serum (FBS) would be most suitable. Any sudden changes undergone by serum-free cultures will most probably induce considerable stress in cells, potentially causing them to alter their EV secretion.6
EV-depleted FBS media require long ultracentrifugation processes to remove FBS-derived EV contaminants for purified EV depletion. However, recent research shows that extending the ultracentrifugation protocol cannot produce a fully contaminated-free medium. Problematically, any remaining bovine small RNA can be mistaken for human RNA.7
Further factors that must be thoroughly considered in EV cancer therapy protocols include:
- Cell culture matrices and plastics
- Exact cell culture medium
- Cell passage, confluency, and viability
- Mycoplasma-status
- Other microbial contaminants6
GMP guidelines for EV cancer treatment research
To comply with good manufacturing practices (GMP) in EV cancer treatment research, you must follow the GMP grade production method for exosomes. This advises you on cell types, culture environments, cultivation systems, and cell culture media. After EV production, a further 3-step purification protocol is required.8
Once purified, a method must be chosen to fully identify and characterize the exosomes via their physical properties and bioactivity. European Medicines Agency provides scientific recommendations on classifying advanced therapy medicinal products. This agency supports scientific progress in cellular biomedicine to advance disease therapies, offering guidelines, recommendations, and criteria to help researchers advance their medicinal/therapeutic products and improve directive regulation.
References
- Lin, W. and Cai, X.D. 2021. Current strategies for cancer cell-derived extracellular vesicles for cancer therapy. Frontiers in Oncology. 11, p.758884.
- Pegtel, D.M. and Gould, S.J. 2019. Exosomes. Annual review of biochemistry. 88, pp.487-514.
- Kosaka, N., Yoshioka, Y., Fujita, Y. and Ochiya, T. 2016. Versatile roles of extracellular vesicles in cancer. The Journal of clinical investigation. 126(4), pp.1163-1172.
- Kok, V.C. and Yu, C.C. 2020. Cancer-derived exosomes: their role in cancer biology and biomarker development. International Journal of Nanomedicine. pp.8019-8036.
- Meng, W., He, C., Hao, Y., Wang, L., Li, L. and Zhu, G. 2020. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug delivery. 27(1), pp.585-598.
- Allelein, S., Medina-Perez, P., Lopes, A.L.H., Rau, S., Hause, G., Kölsch, A. and Kuhlmeier, D. 2021. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Scientific Reports. 11(1), p.11585.
- Mannerström, B., Paananen, R.O., Abu-Shahba, A.G., Moilanen, J., Seppänen-Kaijansinkko, R. and Kaur, S. 2019. Extracellular small non-coding RNA contaminants in fetal bovine serum and serum-free media. Scientific Reports. 9(1), p.5538.
- Rezaie, J., Feghhi, M. and Etemadi, T. 2022. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Communication and Signaling, 20(1), pp.1-13.
