Our custom cell culture media solutions include products that are tailored to your needs. We own our entire manufacturing process, so we can create customized products for clients in a huge range of markets worldwide.
We empower scientists to conduct world-class research by offering an extensive portfolio of human primary cells, stem cells, blood cells, and optimized cell growth and differentiation media. In addition, we are dedicated to supporting you by providing specialized media for your specific research areas, such as 3D cell culture and in vitro cancer applications.
We can fine-tune our flexible manufacturing process to offer custom cell culture media that meet your research and manufacturing needs, regardless of the organization’s size and stage of your research.
Our certifications
 PromoCell operates according to ISO 9001:2015 in order to consistently provide products and services that meet customer and applicable statutory and regulatory requirements.
PromoCell operates according to ISO 9001:2015 in order to consistently provide products and services that meet customer and applicable statutory and regulatory requirements. Our Quality Management System is certified accordig to the EXCIPACT™ GMP standard.
Our Quality Management System is certified accordig to the EXCIPACT™ GMP standard.
Customized cell culture media
As we own our media manufacturing process, we can supply you with our custom media service individual media modifications and additional QC tests, GMP support and custom tailored batch sizes.
Media solutions according to your needs
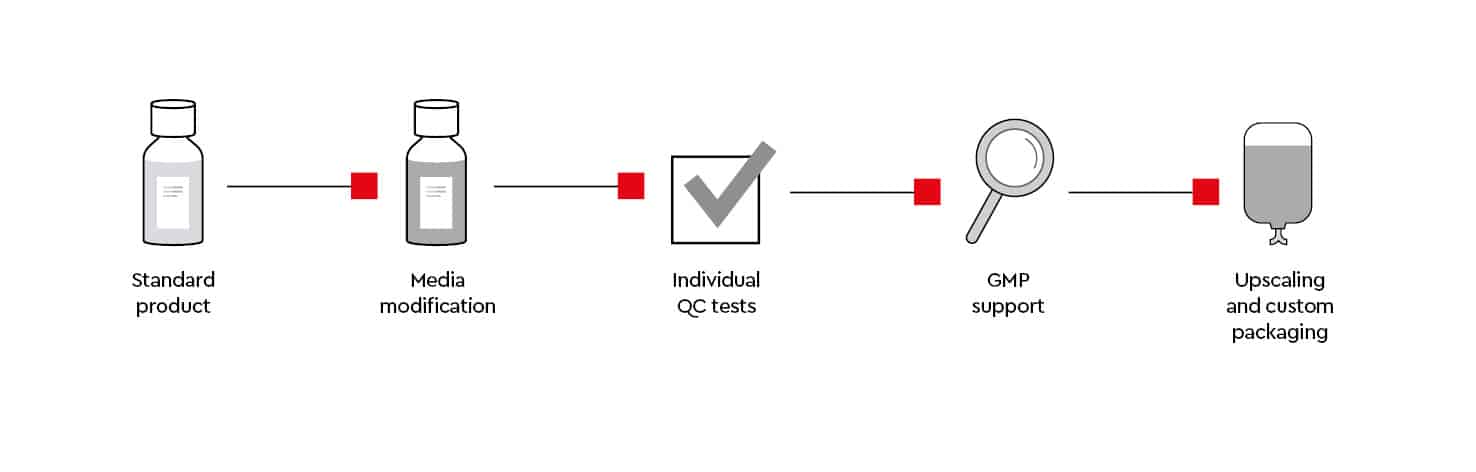
Media modification requirements
We can support your research by providing custom cell culture media formulations (e.g., without glucose or specific amino acids, SILAC) that meet your research requirements.
Individual QC tests
If required, we can offer additional quality tests or documentation (e.g., functional tests, endotoxins) that are not covered in our standard quality control process.
EXCiPACT™-certified GMP standard
For your regulatory requirements we offer our custom cell culture media solutions that are manufactured in compliance with EXCiPACTTM GMP certification standard. For our GMP grade custom cell culture media we proactively inform you about changes in raw material specification, which may impact the regulatory status. With our cell culture media, you will receive the following documentation necessary for your risk assessment and regulatory purposes:
- EQAF (Excipient Quality Assessment File)
- Statement of GMP compliance
- COA (Certification of Analysis)
- COO (Certification of Origin)
- Statement of GMO (Genetically Modified Organisms)
- Declaration of Conformity
- Statement on BSE/TSE Status
- Statement on MVS (Microbial and Viral Safety)
To support the transition from assay development to GMP compliant manufacturing, we also offer a standard version for all media produced in a GMP regulated environment.
Upscaling and custom packaging
Do you require large quantities of media or customized packaging?
We produce large customized cell culture media batches in bigger packaging formats containing the corresponding media required:
- Media bottles (e.g., 1L, 2L)
- Media bags (e.g., 2L, 5L, 10L, 20L)
We also offer custom or bulk growth supplements (e.g., bulk BPE and ECGS) to help you upscale your research projects.
Contact us for more information
Fill out our media request to let us know your individual needs. We can provide you with a customized cell culture media solution based on your requirements.
