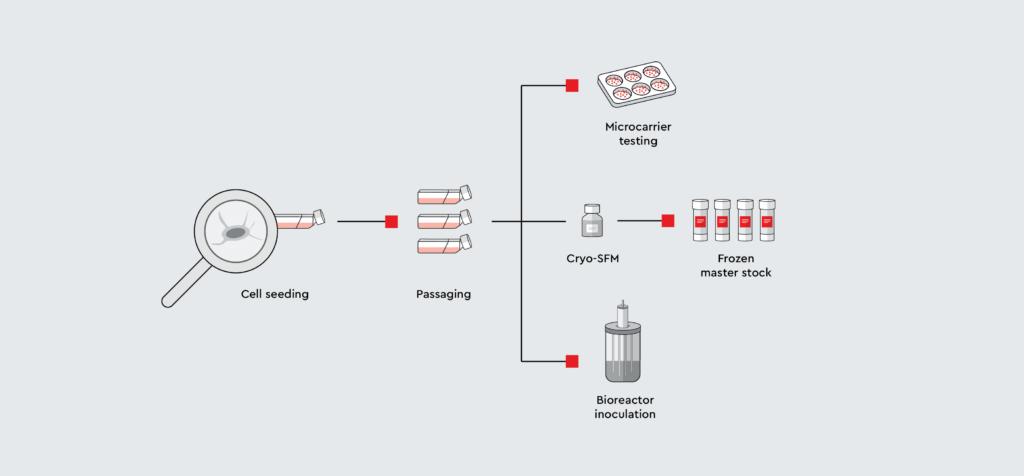
Mesenchymal stem cells (MSCs) are a relatively rare cell population, and large quantities of cells are typically needed for clinical applications. This creates the need for large-scale platforms to efficiently expand MSCs.
Learn why bioreactors are a useful tool for upscaling MSC cultures and how to use them for Good Manufacturing Practice (GMP) compliant large-scale production of MSCs in this protocol.
The app note contains:
- Background information on this topic
- An easy-to-understand bioreactor MSC expansion protocol using our MSC Growth Medium XF
- Results regarding attachment and cell expansion
- Results of the metabolite analysis
