The choice of a suitable culture medium is a crucial factor for the in vitro cell cultivation and significantly affects the success of cell culture experiments from the first step of development to the transitioning to clinical applications.
We are experiencing a rapidly growing demand from the biopharmaceutical industry planning to work with our products in a regulated environment and therefore searching for customized regulatory support. Understanding and evaluating the various raw material risks of our media and reagents is an important step for researchers enabling the transition of cell therapies from development to clinical stages and subsequent commercial manufacturing.
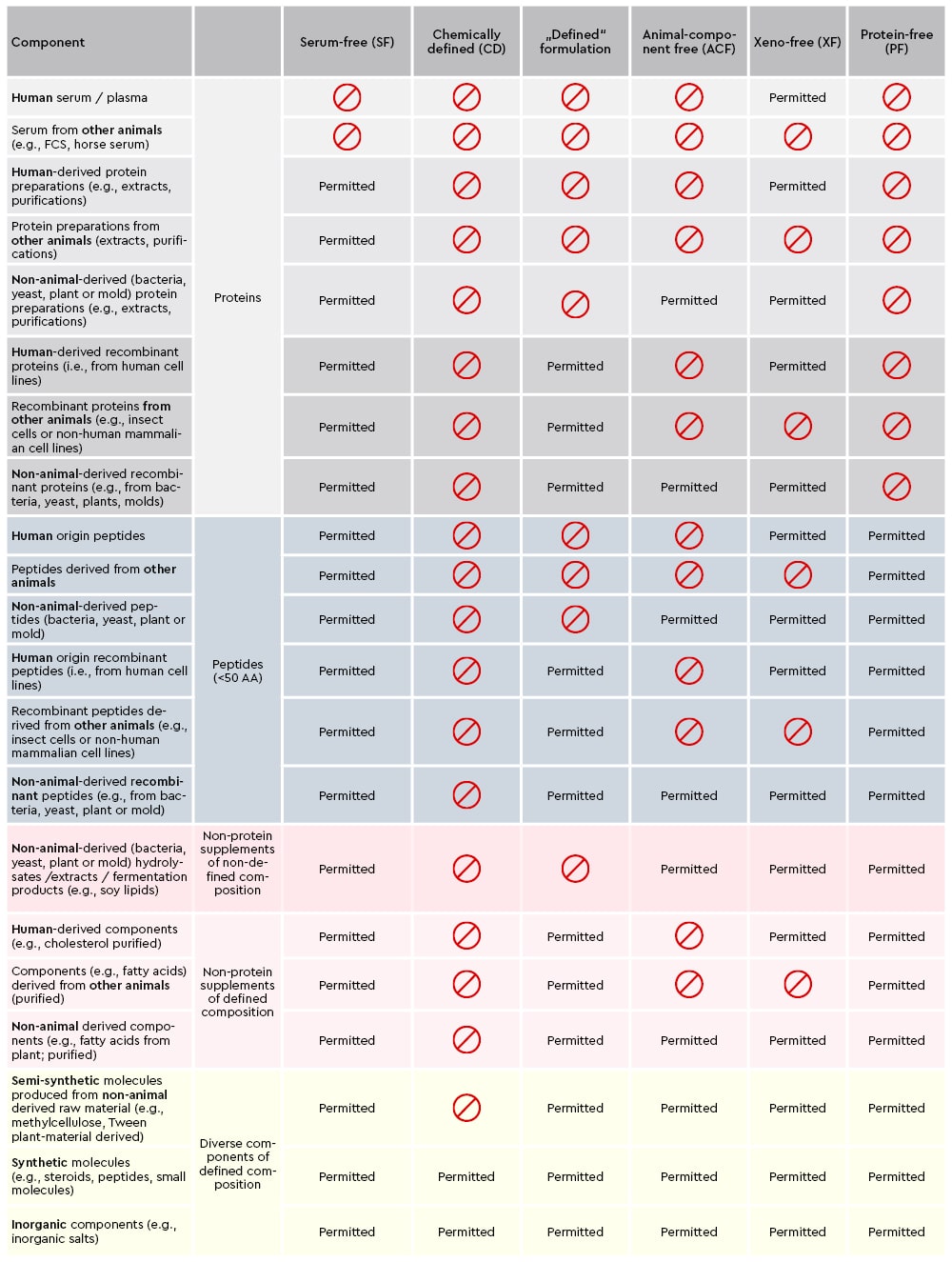
Due to the different requirements of primary cells and the researcher’s applications, we provide a wide range of advanced media formulations. Thereby, it is essential for researchers to have a clear understanding on how to define the specifications of our cell culture media and reagents, from serum-free or xeno-free to chemically defined, to estimate and understand the associated implications for their intended applications.
With a clear communication regarding the definition of raw material specifications and our EXCiPACT™ GMP certification scheme for pharmaceutical excipients we aid researchers in choosing the right cell culture environment and support them with their specific custom regulatory requirements.
Click here to download the Guide.
References
Solomon J. et al. Cytotherapy. 2016 Jan;18(1):1-12.

