A cell culture is a world of its own. The culture dish contains nutrients within the media that cells need to thrive and prosper. But every now and then, unwanted intruders sneak into the cultures and endanger the fragile balance of the system, spoiling the scientific results. In the end, the cultures are useless and must be discarded. But how can we keep these invaders out? One answer for many scientists is using antibiotics. These compounds are the frontline therapy in the fight against bacterial contamination.
Cell culture: a complex microcosm
Cell culture is one of the most common and complex technologies in the life sciences and provides an excellent model for investigating physiological and pathological processes, as well as the effects of drugs and toxic compounds at the cellular and subcellular levels. Cell culture is also used for large-scale production of vaccines, therapeutic proteins and cells for regenerative medicine. The media utilized in various laboratory settings represent a critical element for maintaining healthy, proliferating cells. One fundamental requirement of culturing cells in vitro is avoiding microbiological contamination.
This is why standard cell culture protocols often include the prophylactic use of antibiotics, such as penicillin, streptomycin, gentamicin or amphotericin as media supplements to reduce infection rates. Relatively little is known, however, about the effects of these substances on the metabolism of cultured cells, cell proliferation, differentiation or gene expression. Do antibiotics indeed help to solve the contamination issue, or are they creating additional new problems? “The main goal to use antibiotics in cell culture is to kill bacteria or inhibit their proliferation,” explains Dr. Muna Ali, a scientific support specialist at PromoCell. “However, it is easy to forget that they can also harm the cells themselves as they cause many side effects: For instance, antibiotics can attack other specific, non-bacterial structures in the cell.”
Antibiotics deeply influence cell metabolism
From the 1970s to the 90s, numerous reports presented evidence of the antiproliferative effects of beta-lactam antibiotics like penicillin on cultured eukaryotic cells (Neftel et al., 1987). Similar findings were obtained when using aminoglycosides such as streptomycin or gentamicin (Fischer et al., 1975, Cooper at al., 1990, Cooper et al., 1991). Nevertheless, most research groups continued to incorporate antibiotics in their culture media. “One reason why researchers forget about the side effects of antibiotics is that they are not always obvious.
This is especially true when using immortalized cell lines, which proliferate indefinitely,” explains Ali. However, recent advances in regenerative medicine, and the increasing use of cultured cells for therapeutic approaches, have prompted investigators to better evaluate the influence of antibiotics on the biochemistry and differentiation potential of human cultured cells rather than cell lines. Llobet and colleagues described how the mix of penicillin/streptomycin or gentamicin alone can affect the differentiation of human adipose-tissue derived stem cells into adipocytes. Similar effects were observed on embryonic stem cells (Cohen et al., 2006, Varghese et al., 2017), mesenchymal stem cells (Chang et al., 2006), primary cancer cell lines (Relier et al., 2016) and keratinocytes (Nygaard et al., 2015). The use of antibiotics can also significantly alter gene expression and regulation (Ryu et al., 2017) and could modify the results of studies focused on drug response, cell cycle regulation and cell differentiation.
Negative effects of antibiotics on human keratinocytes
Normal human epidermal keratinocytes (NHEK) are widely used in the field of basic research, and conventional monolayer or three-dimensional cultures have been the standard approach for almost 30 years. Beta-lactam antibiotics, as well as aminoglycosides, reduce the proliferation rate of NHEK and hinder the development of a fully differentiated epidermis in 3D skin models (Nygaard et al., 2015).

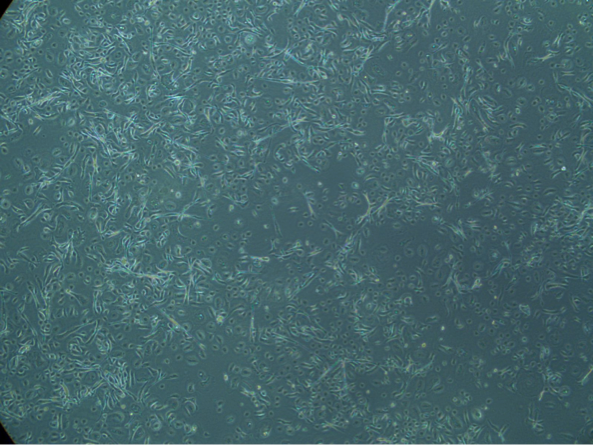
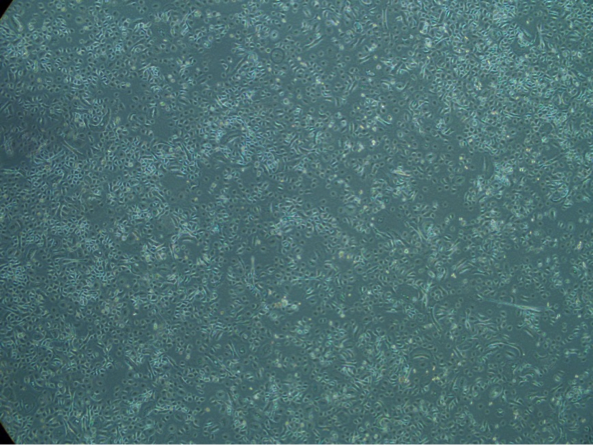
One researcher whose work depends on cultured primary keratinocytes without contamination is Dr. Thomas Haarmann-Stemmann who works at the Leibniz Research Institute for Environmental Medicine in Duesseldorf. He is researching an explanation of the molecular mechanisms of UV-induced cell damage. His long-term aim is to develop new strategies for the prevention of environmentally-induced skin diseases. “We use primary skin cells as keratinocytes, fibroblasts, and melanocytes, as well as cell lines,” he explains. “We add antibiotics to our culture media for preventing bacterial contamination and in gene expression studies. However, when culturing NHEK in the presence of antibiotics, we observed a marked decrease in their proliferation rate.”
After an exchange of ideas with Dr. Ali at PromoCell, Dr. Haarmann-Stemmann better characterized this phenomenon by culturing the cells with and without antibiotics. In a parallel test, NHEK from the same batch were cultured with and without gentamicin and amphotericin under identical conditions in Duesseldorf. The same cell batch was also cultured in the PromoCell laboratories according to their specific guidelines, which recommends avoiding antibiotics in the culture medium.

“After four to five days of culture, we could see a clear difference in growth rate. Whereas NHEK without antibiotics had filled up the culture dish, the cells just showed a 30%-40% confluence in the presence of gentamicin and amphotericin,” remarks Haarmann-Stemmann. “The keratinocytes cultured at PromoCell performed equally to their counterparts in Haarmann Stemmann’s lab but only when treated without antibiotics. The parallel test showed conclusively that cells treated with antibiotics grow slower and stop proliferating earlier,” says Ali.
Similar observations are reported by Sabine Falk, a laboratory technician at Merz Pharmaceuticals, a Frankfurt, Germany-based international company working in the field of aesthetics and neurotoxins. “We are performing cell-based assays using keratinocytes, fibroblasts and adipocytes instead of conducting animal tests. When culturing NHEK, we add penicillin and streptomycin to avoid microbiological contamination. However, we realized that the cells showed a poor growth rate and adherence to the culture dish in the presence of antibiotics.” Parallel tests like the ones in Haarmann-Stemmann’s lab also confirmed these findings: The adverse effects of antibiotics might be caused by their negative influence on mitochondrial functions. According to the endosymbiont theory, mitochondria are of bacterial origin, and their molecular and structural components are very similar (Singh et al., 2013).
In sum: Try to exclude antibiotics from your cell culture
“The above-mentioned findings strongly confirm PromoCell’s recommendation of avoiding the use of antibiotics in cell culture,” concludes Ali. “Most primary or normal human cells show reduced growth rates in the presence of antibiotics. Keeping the cells free from microorganism contamination can be accomplished with proper knowledge of good laboratory practice. Following all the guidelines towards a sterile technique makes these compounds unnecessary.” Aseptic techniques, including a sterile work area, sterile reagents and media, good personal hygiene and sterile handling, act as a barrier between bacteria in the environment and the sterile cell culture (Stacey, 2011).