
3D Cell Culture
Find suitable products, scientific resources or answers to your questions within our technical library FAQ.
Checkout using your account
This form is protected by reCAPTCHA - the Google Privacy Policy and Terms of Service apply.
Checkout as a new customer
Creating an account has many benefits:
Immuno-oncology is a unique combination of oncology and immunology. By activating or amplifying the body’s natural defenses, immuno-oncology aims to boost the immune system to fight cancer better. Targeted antibodies or immunomodulators are well-known immunotherapies; adoptive cell therapy using CAR-T or TCR cells is particularly important, representing a significant breakthrough in cancer therapy with the recent approval of several CAR-T therapies.
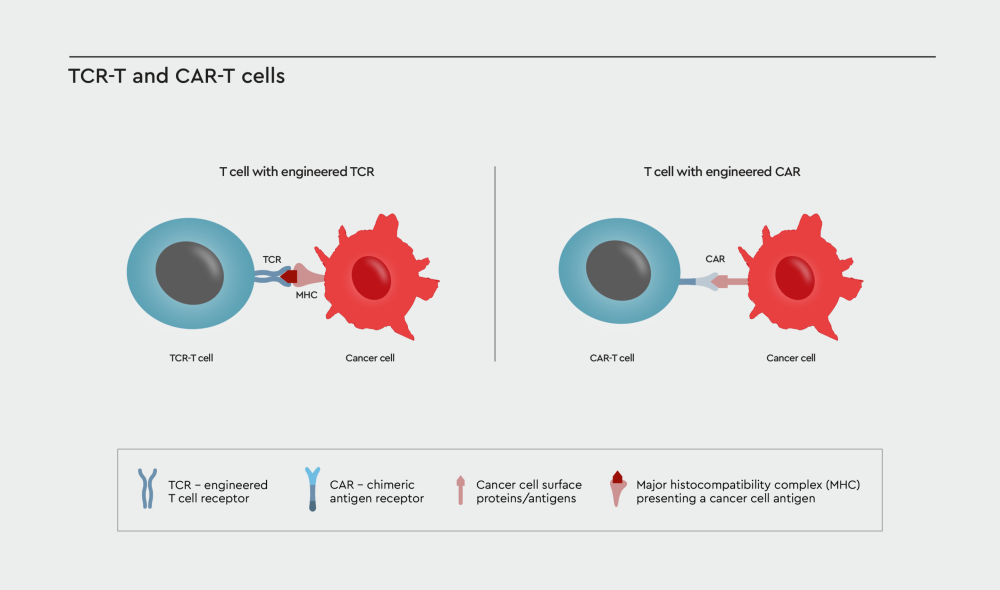

Figure 1: TCR- or CAR-engineered T cells. T cell receptor (TCR)-engineered and chimeric antigen receptor (CAR)-T cells both redirect the T cell against a specific target on the cancer cell surface. However, the two therapeutic approaches differ in the way the T cell receptor is designed. TCR-T cells are modified by transferring a naturally occurring T cell receptor into the cell that specifically recognize tumor antigens presented by the major histocompatibility complex (MHC). In CAR-T cells the TCR is replaced by a new receptor that utilizes a fragment of a human or mouse antibody to recognize specific tumor associated-antigens on the cancer cell surface.
Existing preclinical models failed to predict severe toxicities seen in human clinical trials after engineered T-cell infusion. Furthermore, 2D cancer cell-based assays are insufficient to determine the mode of action to predict immunoreactivity and efficacy before moving to in vivo models and eventually into the patient. Relevant, reliable, and scalable 3D in vitro assays are needed to enhance the development of immuno-oncology therapies.
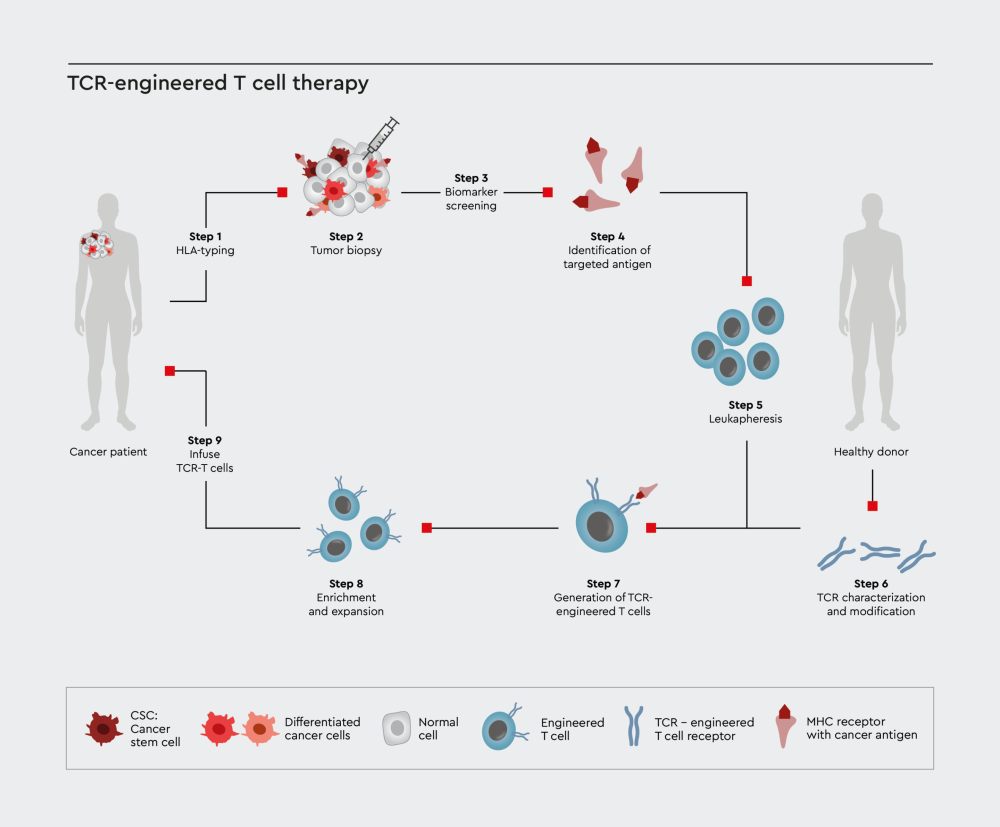

Figure 2: Schematic illustration for the immuno-oncology therapy process using T cell receptor (TCR) approach.
Researchers use sophisticated methods to identify tumor antigens and design antibodies or effector cells (e.g., CAR-T or TCR cells). But less sophistication is applied to when choosing the appropriate tumor models. Obviously, choosing an inadequate tumor model puts the drug development on a misleading path.
We offer a toolbox of cancer media to build high-quality cancer assay panels with patient-derived organoids (PDOs) to model the immunotherapy function. These cancer media also ensure the support of cancer stem cells. We illustrate how to use our toolbox for a 3D in vitro assay strategy in the following sections.
In addition, we offer a wide panel of pre-HLA-typed primary cells from all relevant tissues and media for non-cancer cells.
Find out more about our HLA-typed Human Primary Cells
Recently, a bi-specific T cell engager was approved for the treatment of uveal melanoma–another example of how immuno-oncology-based therapies can be employed (U.S. Food & Drug Administration, 2022). The enduring challenge, however, is how to target solid tumors. While CAR-T therapies have demonstrated clinical efficacy for treating primarily B cell malignancies, TCR-based therapies are regaining interest. We show here an example of how to use our cancer media toolbox for efficacy testing of engineered T cells against a patient-derived spheroid model using our 3D Tumorsphere Medium XF.
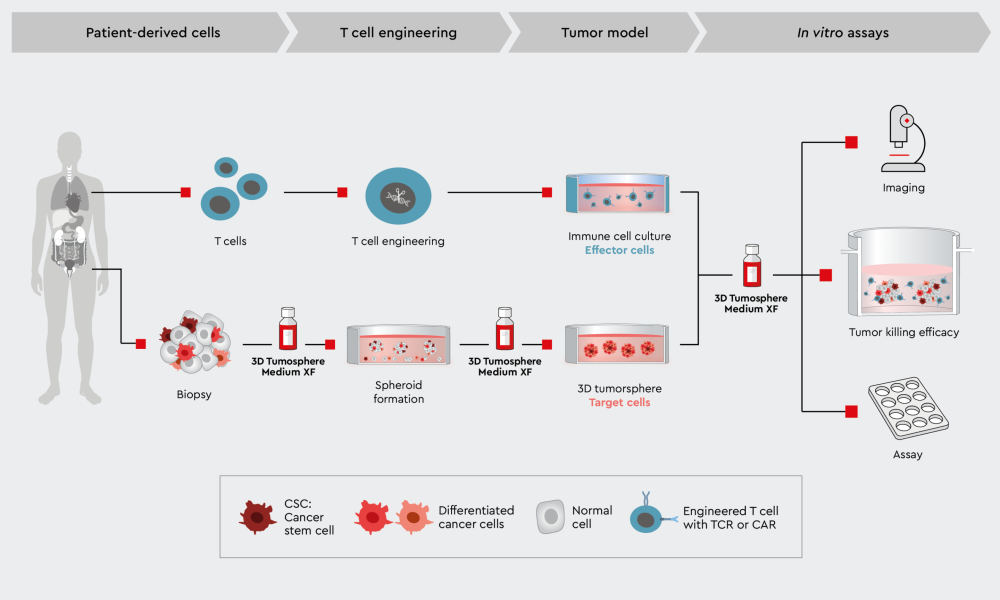

Figure 3: Workflow to characterize CAR-T cell tumor killing efficiency in a 3D cancer model. Example of a tumor killing efficiency workflow using the 3D Tumorsphere Medium XF to develop the primary target cancer model.
The 3D Tumorsphere Medium XF can be used in plates with or without a coating. Also, extracellular matrix components and reagents can be used combinatorially. To learn more about how to develop organoids with several cell types with the cancer media toolbox, read further about our application side tumor microenvironment. Again, the key point to preserve tumor traits in vitro is the maintenance of cancer stem cells. This increases the relevance of the model and the predictiveness of data when moving into in vivo models.
Please note: all media of the toolbox are growth media that can keep the cancer model over several passages and can therefore be used to establish primary cancer cell lines or to freeze organoids.
Streamlining the evaluating of on-target, off-target, and on-target/off-tumor effects
The above example describes the first step in drug candidate evaluation: the determination of its tumor killing efficacy through the desired target using a 3D tumor model. The advantage is obvious. While 2D cancer cell assays are suitable for determining on-target efficacy, these assays fail to recapitulate the complexity of tumor biology. 3D models such as spheroids, organoids or tumoroids are better suitable and can mimic the tumor microenvironment (TME). Even more, since the TME helps cancer cells to evade the immune response, for which the drug candidate was actually designed for. In this section we describe, how to expand such 3D assay configurations to a panel of assays addressing also off-target and on-target/off-tumor activity using our toolbox of different cancer media:
The media of our toolbox support the development of organoids with different levels of complexity, that
The media can be combined within an assay workflow or even modularly within an assay and are suited for most tumor types. The design of the media ensures the maintenance of cancer stem cells, which drive the tumor development.
Furthermore, our media are synthetic media without serum and contain all nutrients to keep cells or even passages in a healthy state. This enables further medium components such as extracellular matrices or assay specific reagents.
The second step establishes high selectivity of the drug candidate against its target in a normal cell (on-target/off-tumor) to reduce the T-cell therapy-associated toxicity. The following figure illustrates a possible and new 3D in vitro assay panel strategy.
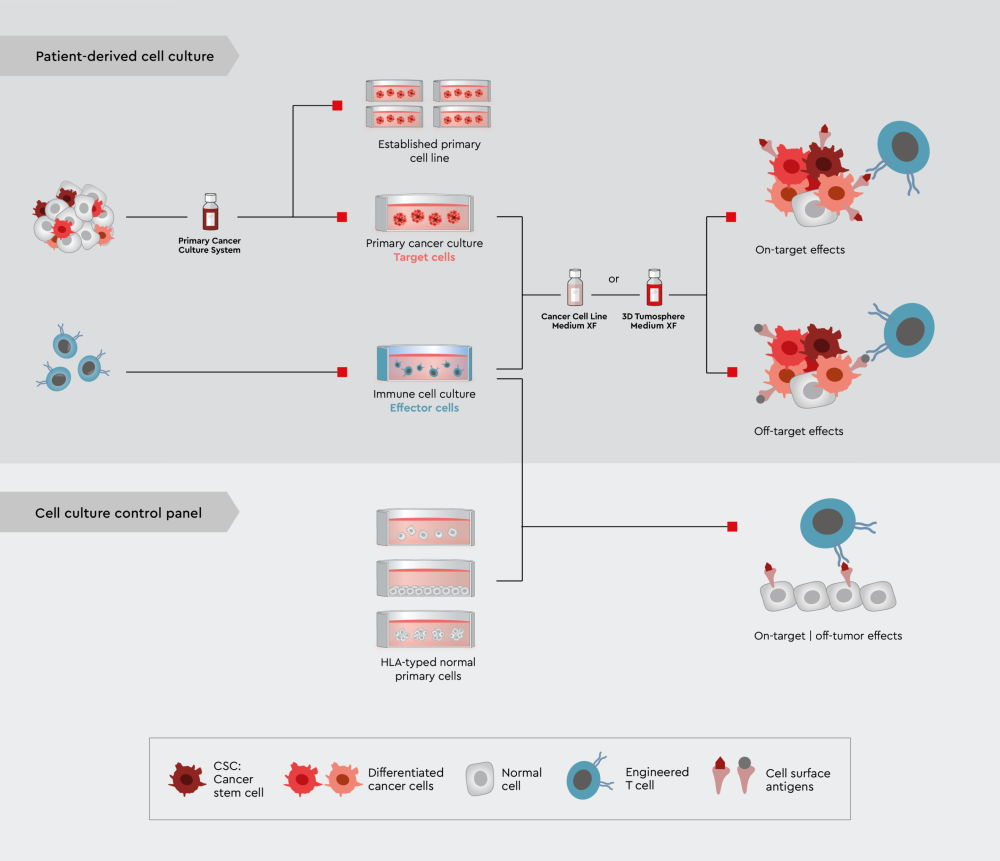

Figure 4: A novel in vitro assay panel strategy using tumor trait preserving media for organoids to characterize drug candidates for immuno-oncology.
Upper panel: Efficacy and selectivity testing employing 3D tumor models or PDO. Lower panel: On-target/off-tumor testing employing various non-cancer cell lines, ideally non-cancer 3D models or even organ-chip approaches.
Have a look at other Research Areas
PromoCell uses HubSpot to provide LiveChat support. This feature is currently blocked due to your cookie preferences.
Accept cookies to enable LiveChat