Selected as the 2013 “Breakthrough of the Year” by Science magazine, cancer immunotherapy shows a completely new way to treat malignancies by using the body’s own immune system, and not by targeting the tumor itself. This cutting-edge area of research and therapy has shown very promising results. However, further investigation is needed to turn immunotherapy into a cancer therapy that can be broadly applied.
- Sometimes they do not display enough differences to be recognized, as they develop from “normal” cells.
- Sometimes they get caught by the immune cells, but the response is not strong enough to destroy them.
- Sometimes they release substances that hinder the immune response.
Antibody that is attached to a cell.
Cancer immunotherapy: a fragile balance between fighting cancer and preventing autoimmunity

Teaching the immune system how to fight cancer:
Prof. Dr. Thomas Wölfel from the University Medical Center Mainz has been studying the interactions between cancer cells and immune systems for over 30 years. Picture kindly provided by Professor Thomas Wölfel.
Checkpoint inhibitors: releasing the brakes on T cells
The balance between stimulatory and inhibitory signals is crucial for avoiding uncontrolled immune reactions that would damage the body. Therefore, immune cells have immune checkpoints on their surface. Those receptors – when binding to the ubiquitous ligands on body cells – prevent them from attacking “normal” cells. Tumors can also activate suppressive immune checkpoint pathways that minimize the immune response. In the late 1990, James Allison, a cancer immunologist at the University of Berkeley in California, found that the administration of antibodies at one of these checkpoints, the cytotoxic T-lymphocyte associated protein 4 (CTLA-4), eliminated tumors in mice (Leach DR et al., 1996).
Cancer cells under attack:
Immune cells usually track down and destroy malignant cells. But some tumors resist such attempts and evade the reaction. © Christoph Burgstedt / Shutterstock.com
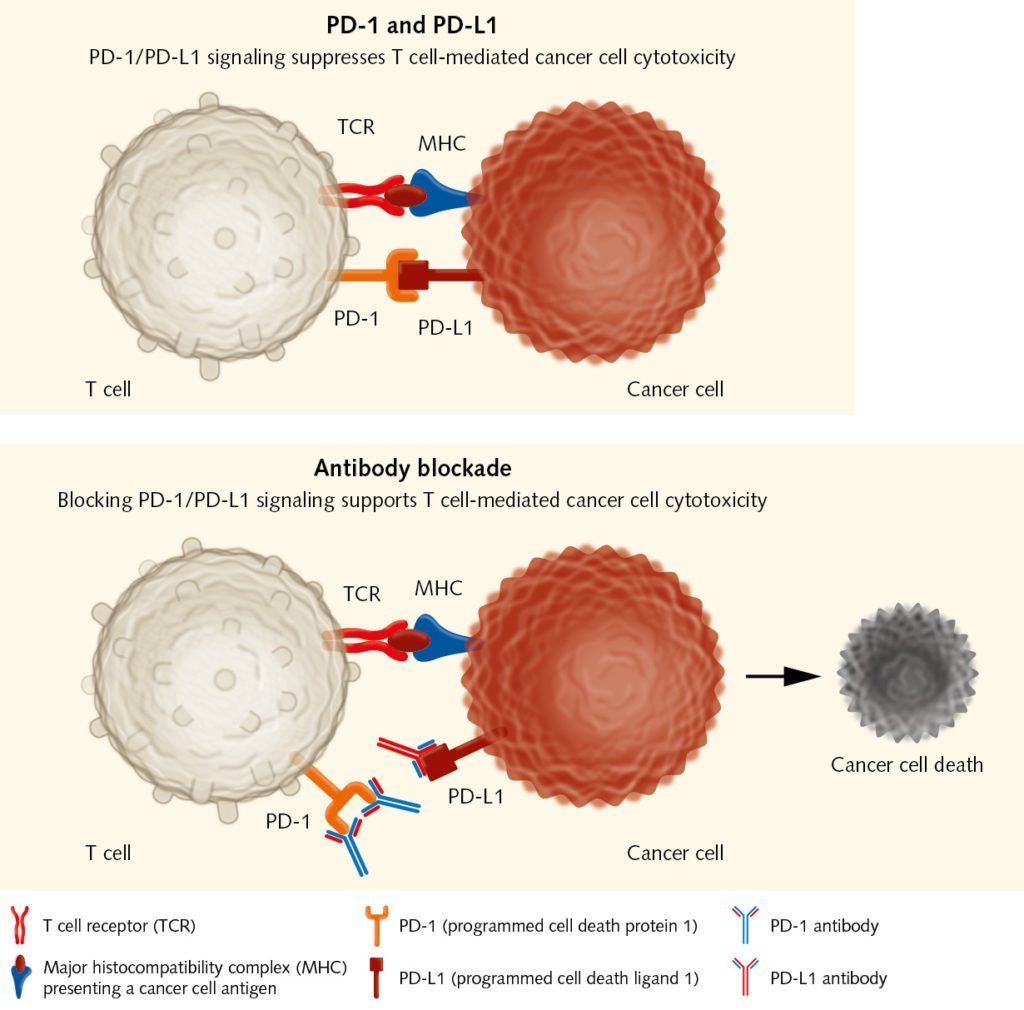

Releasing the brakes on T cells:
Immune checkpoint inhibitors as PD-1 antibodies allow tumor-reactive T cells to kill cancer cells.
Cancer vaccines: stimulating the immune system to destroy cancer cells
Another approach that targets the early steps in the cancer-immunity cycle is the vaccination with tumor-associated or tumor-specific antigens. The purpose of cancer vaccines is to initiate and boost an antigen-specific T cell response when the tumor has failed to do this. Advances in technology have led to the identification of many antigens that can be targeted in cancer immunotherapy. These antigens are introduced into the patients, where they stimulate effector T cells to selectively attack cancer cells that express them. There are several ways to vaccinate the patients. Dendritic-cell vaccines are considered among the most successful approaches so far, as these cells can elicit strong immune responses (see text box). Other vaccination approaches use whole tumor cells, DNA or RNA, peptides or proteins, as well as viruses (Kamta, et al., 2017). “There is a high interest in finding neoantigens, which arise from mutations within the cancer genome, and which can elicit a T cell response,” remarks Wölfel. “In recent years, new high throughput DNA-sequencing methods have allowed the identification of antigens in individual patients in an acceptable period of time. Nevertheless, the mean time of approximately three months might be too long for some patients in an advanced stage of the disease without other therapeutic options.” Despite researchers’ high expectations, early clinical studies of vaccines did not show the anticipated results, as cancer-mediated T cell suppression prevented T cell activation. Therefore, recent studies have actively focused on combining vaccination with other approaches such as T cell checkpoint inhibition (Patel et al., 2016). Improving vaccine design, and using vaccines to increase the effects of standard therapies could also maximize therapeutic benefits for cancer patients. [info_box headline="Dendritic cell based vaccines"] Dendritic cells (DCs) are the most powerful antigen-presenting cells in the human body. They take up foreign molecules, process them and present them to T cells starting an immune response. During the past decade, DCs have been the subject of numerous studies aimed at gaining a better insight into their immunobiology, their interaction with both the innate and adaptive immune system, and their role in the tumor microenvironment. In the context of cancer immunity, DCs can activate tumor-specific T cells in lymphatic tissues. This is why DCs can be used as adjuvants for vaccination, and why they hold great promise for the treatment of cancer (Shang, et al., 2017). Sipuleucel-T is so far the only FDA-approved DC-based vaccine. It is used for treating advanced prostate cancer, as it has shown a survival benefit for patients. “Dendritic cells are essential for inducing an immune reaction,” explains Prof. Dr. med. Thomas Wölfel from Internal Medicine III of the University Medical Center Mainz. “The question is how they should be used therapeutically. Should they be extracted from the body, loaded with the relevant antigen and then reinfused, or should they be activated in the body through so-called danger signals?” Formulated in 1994 by Polly Matzinger, the danger model postulates that the immune system differentiates between “dangerous” and “safe” by recognizing alarm signals coming from injured tissues. These danger signals can activate DCs and initiate an immune response. If tumors do not send those signals, they remain invisible to the immune system. For this reason, the efficacy of DC vaccines is strongly influenced by the method of administration. Low numbers of DCs at the tumor site, impaired DC maturation, or the presence of inhibitory signals can endanger the therapeutic efficacy of the vaccine. Ex vivo DCs are mainly generated from bone marrow progenitor cells in the presence of GM-CSF and IL-4 or IL-13 (Pizzurro et al., 2015). In vivo vaccination strategies, where DCs are directly activated at multiple sites, offer a cheaper and simpler approach. To optimize DC-based vaccination strategies, researchers are now focusing on determining the optimal tumor-associated antigens to be used, the best way of administration, as well as possible combination therapies.[/info_box]Cell therapy: providing the immune system with the missing effectors
The aim of immune checkpoint inhibitors and cancer vaccines is to trigger pre-existing immune responses. Yet what happens if there are no or not enough effector cells to fight the tumor? To overcome this deficit, researchers can extract T cells from a patient’s blood or tumor samples, expand them in vitro, and reintroduce them into the patient as a cancer therapy. Steven Rosenberg had been working for years on the adoptive transfer of tumor infiltrating lymphocytes (TILs), when in 2010, he published encouraging results obtained with the so-called chimeric antigen receptor therapy (CAR). Here, a patient’s T cells are collected and genetically engineered so that they target cancer cells (June et al., 2018). CAR therapy holds significant promise for the future.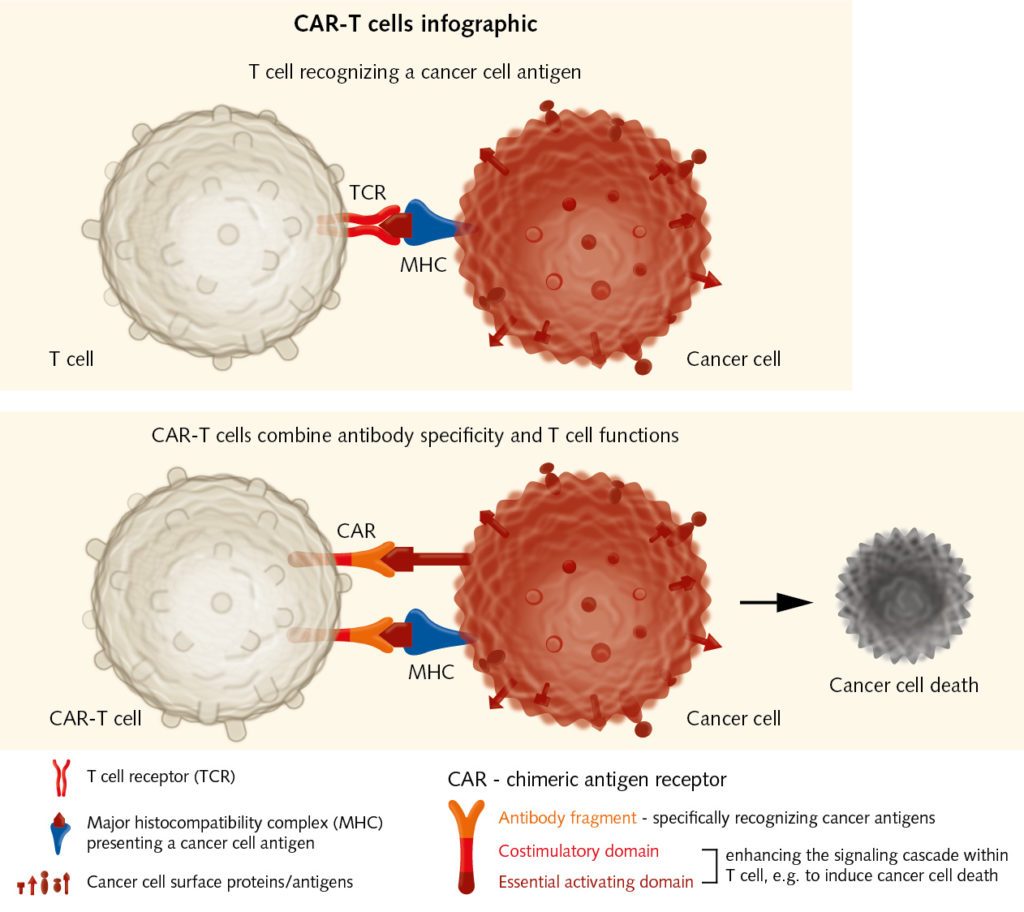
Adoptive transfer of effector T cells:
Chimeric Antigen Receptor immunotherapy has shown good results in the treatment of hematological malignancies. It could also be used for solid tumors. CAR-T cells combine recognition specificity of antibodies with T cell functions, e.g. cytotoxicity, and thereby enhance immune responses against cancer cells.
Future challenges: finding the right approach for each patient
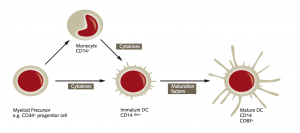
Maturation scheme of myeloid dendritic cells:
The scheme stems from our App Note “Generation of monocyte-derived Dendritic Cells (moDCs).”
Basics of cancer immunotherapy
In late 2017, Professor Thomas Wölfel summarized recent advances in cancer vaccination. He points to various exciting opportunities and innovative methods arising from recent research breakthroughs. And progress continues, with the emerging field of predictive biomarkers bringing individually suited therapies one step closer. Read about Professor Thomas Wölfel's perspectives on the future of cancer vaccination.
FIND OUT MORE
Related resources
Immunotherapy: Boosting the immune system to fight cancer:
https://www.youtube.com/watch?v=-NNjDjXSJt0
Which Cancers Can Be Treated With Immunotherapy? Ask a Scientist.
https://www.youtube.com/watch?v=BiJFmpfpcLc


