
3D Cell Culture
Find suitable products, scientific resources or answers to your questions within our technical library FAQ.
Checkout using your account
This form is protected by reCAPTCHA - the Google Privacy Policy and Terms of Service apply.
Checkout as a new customer
Creating an account has many benefits:
Isolating patient-derived cancer cells from different types of biopsies (bone marrow biopsy, core needle biopsy, endoscopic biopsy, etc.) is the golden standard for cancer research. However, obtaining regular surgical biopsies from patients is a challenging prospect. Additionally, the variability of the quality of tissue samples can be pretty high. Scientists may subsequently spend more time obtaining the right cancer cell population than on the actual experiment. This challenge becomes particularly obvious during assay development when building assay panels to characterize and prioritize drug candidate collections. Investigations and data must be recapitulated over the course of a drug discovery project. How can researchers confirm studies if the biopsy sample is finished?
Challenges establishing assays with patient-derived biopsies:
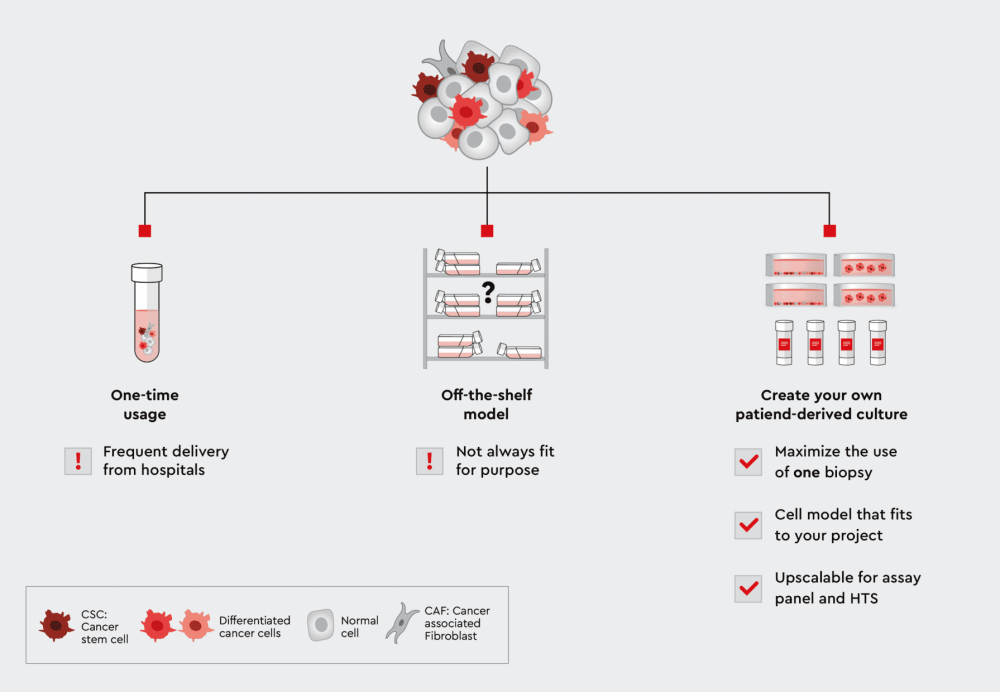

Figure 1: From biopsies to the lab: Three paths to use patient-derived cells for assays. Using the cells only once has the disadvantage that you have to constantly replenish them. On the other hand, if you use an commercially available off-the-shelf model, it may not fit your own individual applications. The solution is therefore an individual patient-derived culture. The patient-derived cell lines allow you to maximize the use of cells from only one biopsy, they fit your application, and the cells are highly scalable for assay panels or high-throughput screening.
Biopsies are highly valuable for health care and research applications. Scientists are trying to make best use of scarce patient material. Losing this material because the derived cells do not develop into a healthy culture or because they have died after a few days is the worst case, particularly when using a personalized cancer therapy approach. But keeping them for a longer period in culture to perform more assays, upscaling to employ sophisticated test panels or building 3D tumor models poses increased requirements to the patient-derived cell culture. In the following section, we offer solutions to facilitate primary cancer cell cultivation and prepare the foundation for advanced assay development strategies.
A toolbox to maximize biopsy use for assay development
We have developed a toolbox of cancer cell media and corresponding workflows to address these challenges. Our toolbox contains three media for cancer cell cultivation. The media can be used individually or in combination, depending on the experimental set up. An essential feature of the media is that they can be used for most cancer cell lines and tumor biopsies, which has been proved in > 30 cell lines and biopsies from different tumor origins. The media design provides a “universal” media toolbox that does not require additional reagents. This makes the choice of media more straightforward and more reliable when working with individual patient samples.
But here is more about the media. The media are functional. That means they are not just keeping cells alive, but they help control the cell population composition.
Advantages of the cancer media toolbox:
Supporting cancer stem cells is the prerequisite for a good assay design. Our media’s underlying mechanism utilizes tumor biology to enhance versatility. Tumor formation is driven by cancer stem cells, which must be present in the cancer cell culture. A healthy cancer stem cell population does a better job for the cell culture than any artificial experimental setting, which may or may not recapitulate tumor biology.
You will find on the “Applications For Our Cancer Media Toolbox” page an overview of applications. In the following sections, we focus on assays for drug candidate testing.
From biopsy to 2D or 3D cultures for assay panels and HTS
When a biopsy has been obtained, examined for quality and dissected, the cells can be directly dispensed in the 3D Tumorsphere Medium XF. From this suspension, spheroids and organoids will form over time. The 3D Tumorsphere Medium XF medium selects for cancer stem cells and cancer cells and will support these cells throughout cultivation. Three paths to an assay are possible:
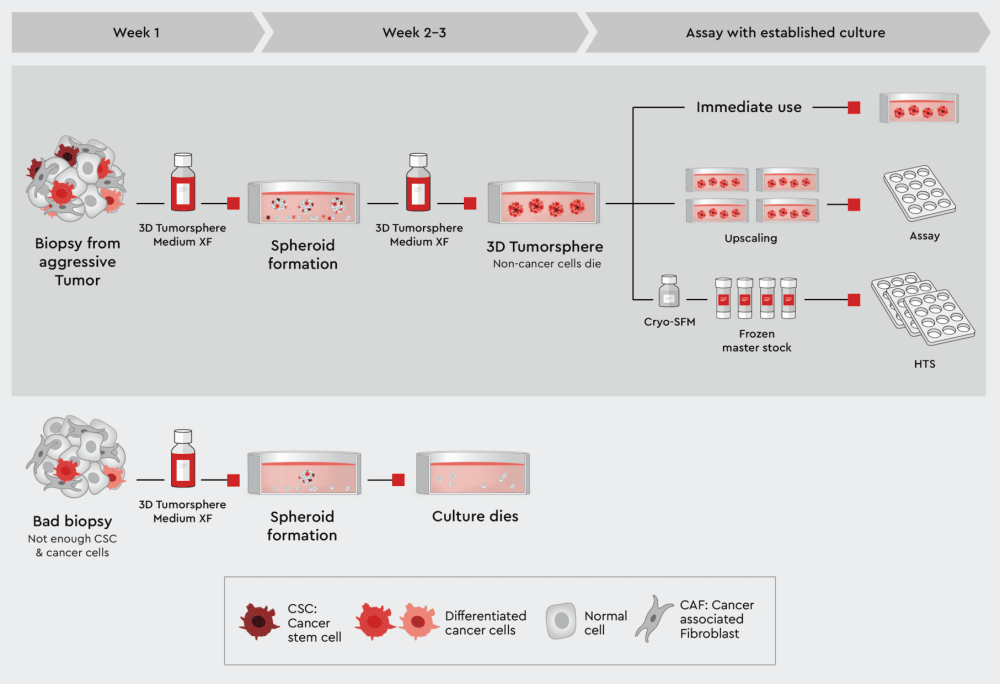

Figure 2: Principal workflow for assayable 3D culture from biopsies using our 3D Tumorsphere Medium XF. Good quality biopsies can be directly suspended into the 3D Tumorsphere Medium XF to form tumorspheres. The 3D cancer culture can be used directly in assays, upscaled, when cancer stem cells were originally present to build a panel of different assays, or frozen to generate master stocks, which can be used in the assay battery or even HTS. In case of a bad quality biopsy, the culture will inevitably die.
Sometimes, it might be desirable to maintain all cell types of the biopsy or at least also the cancer-associated cells next to the cancer cells. The Cancer Cell Line Medium XF should be used in this case. This medium supports all cell types, including the cancer stem cells to maintain the tumor biology. Although this is the fastest way to obtain a patient-derived cell culture, we call it quick and dirty, because non-cancer cells may overgrow cancer and particularly the CSCs. Please check Figure 3 and our application note for the unselective isolation of cells from solid tumors or xenografts.
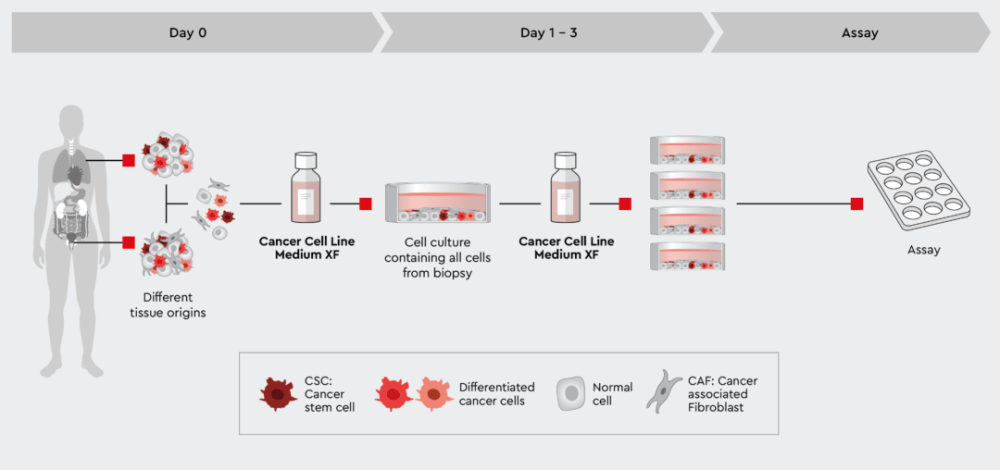

Figure 3: Rapid process from biopsy to assayable culture. The tumor cell preparation can be given directly into the Cancer Cell Line Medium XF. The medium supports all cells no matter what tissue they were isolated from. This quick and dirty approach allows all cells to develop but also maintains cancer stem cells that further drive the development of the culture. Note: if the ratio of non-cancer cells over cancer cells is too high, the culture may be overgrown by non-cancer cells, typically fibroblasts.
Therefore, when designing an assay panel for routine drug candidate testing and optimization or an HTS campaign with a primary cell culture from a biopsy, we recommend using an advanced workflow as described in the next section.
Switching from variable biopsies to your own patient-derived established cancer
When planning to build a panel of assays to test drug candidate collections, optimize leads, or characterize leads in more depth, the number of assays to be performed increases substantially.
Today, two approaches are usually taken. Either continuous supply with biopsies is set up or off-the-shelf models are purchased. Above, we described the issues in the former case. In the latter case, some models would fit the drug discovery strategy. In other cases, the models are unsuitable or not sufficiently described to understand the patient-specific situation from which they originally were derived. A better solution is, to develop your own primary cancer cell lines that fit your purpose.
Until today, the step from the initial biopsy-derived cell culture to a fully established primary cancer cell line was difficult. Now, our cancer media toolbox makes this step much easier and more reliable because the media are designed to isolate cells from biopsies and establish a primary cancer cell line.
Advantages of establishing your own patient-derived primary cell lines:
The media keep cancer cells in culture (2D, 3D or in co-culture) for an extended period of time and over many passages. Furthermore, cultures can be upscaled and cells can be frozen for master and working stocks.
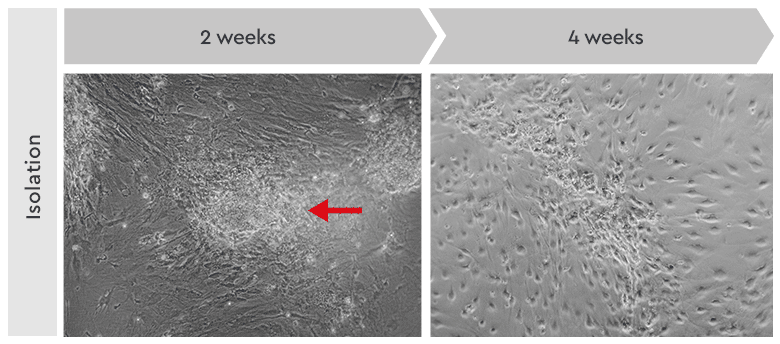

Figure 4: Primary culture derived from an invasive adenocarcinoma. Left: During the first two weeks of the isolation process, the cancer cells appeared as locally restricted and lightly adherent cell clusters (red arrow) on top of the stroma layer. Right: After 4 weeks, highly motile cancer stem cells began migrating from their original locations to cover the whole culture vessel surface. These cells proliferated as a homogeneous population in our Primary Cancer Culture System.
The critical point for maintaining patient-derived cancer cells for upscaling and preparing master stocks is the sufficient presence of healthy cancer stem cells. All three media support CSCs. But whereas the Cancer Cell Line Medium XF supports CSCs, the 3D Tumorsphere Medium XF and the Primary Cancer Culture System actively select for cancer stem cells. This is important, when focusing on drugs against CSCs or on cancer types, which are less aggressive or easily overgrown by fibroblasts. Therefore, we recommend choosing the appropriate cancer medium of the toolbox already in the design phase.
The media share two important features relevant for assay development: they can be used across multiple different cell origins from different cancer types, and they are synthetic (no FCS included):
The nature of the toolbox and the component mixes of each media ensures that the media are compatible to each other. Each medium has a basal medium component that can be used to support cells for up to 48h. This means, when switching from cell culture upscaling to the actual assay, cells can be kept in the basal medium alone for the duration of the assay (assuming typical drug assay protocols of a few hours) and a nutrient density comparable to a minimal medium can be achieved. This reduces interference with ingredients or allows to add assay specific reagents such as specific cytokins, calcium, methycellulose or other factors.
Last but not least, the lot-to-lot variability of the media is small, and without FCS no FCS-batch management is needed facilitating high assay reproducibility and excellent Z’-values.
Have a look at other Research Areas