From bench to bedside and back: This is the direction of malignant mesothelioma research. Yet to understand the biology of this particular type of cancer, more basic insights are still needed. And translating that knowledge into new therapies is extremely important, as only limited therapeutic options exist so far.
 Molecular needles that cross the lung epithelium: That’s how asbestos – often in form of dust – enters lung tissue (Carbone et al., 2012). The term “asbestos” describes a family of silicate minerals that formerly found widespread use in thermal and acoustic insulation in buildings (Westerholm et al., 2017; Allen et al., 2018). Now the substance is banned in many countries. Even decades following exposure, asbestos molecules can lead to cancer, often in the form of a malignant mesothelioma. The correlation between cancer and asbestos was first described in case studies and animal experiments published in the 1940s (Nordmann, 1938; Nordmann and Sorge, 1941; Pira et al., 2018). To understand the pathophysiological mechanisms behind the effects of asbestos and to assess its toxicity, scientists started to use in vitro techniques in the early 1960s (Richmond, 1961). More than 50 years of scientific development followed, and generations of researchers have worked on this topic. However, scientists worldwide are still trying to understand the exact mechanisms behind mesothelioma development as a result of asbestos exposure (Find information on mesothelioma metastasis, reviewed by cancer specialist Dr. James Stevenson, on the website of the Mesothelioma Cancer Alliance).
Molecular needles that cross the lung epithelium: That’s how asbestos – often in form of dust – enters lung tissue (Carbone et al., 2012). The term “asbestos” describes a family of silicate minerals that formerly found widespread use in thermal and acoustic insulation in buildings (Westerholm et al., 2017; Allen et al., 2018). Now the substance is banned in many countries. Even decades following exposure, asbestos molecules can lead to cancer, often in the form of a malignant mesothelioma. The correlation between cancer and asbestos was first described in case studies and animal experiments published in the 1940s (Nordmann, 1938; Nordmann and Sorge, 1941; Pira et al., 2018). To understand the pathophysiological mechanisms behind the effects of asbestos and to assess its toxicity, scientists started to use in vitro techniques in the early 1960s (Richmond, 1961). More than 50 years of scientific development followed, and generations of researchers have worked on this topic. However, scientists worldwide are still trying to understand the exact mechanisms behind mesothelioma development as a result of asbestos exposure (Find information on mesothelioma metastasis, reviewed by cancer specialist Dr. James Stevenson, on the website of the Mesothelioma Cancer Alliance).
Causes of mesothelioma: an unusual mechanism
Using human mesothelial cells from different donors, a group of researchers around Haining Yang analyzed the effect that asbestos has on various types of cells (Yang et al., 2010). Asbestos is cytotoxic, but this feature does not explain carcinogenesis, which requires exactly the opposite, namely unlimited survival of malignant cells. The researchers found an alternative explanation: inflammation. The death of mesothelial cells leads to macrophage recruitment and the secretion of cytokines such as TNF alpha, which results in survival of mesothelial cells and chronic inflammation. “Of course, no one in the scientific community can say with certainty that every case of mesothelioma ever was caused by asbestos, but the majority of cases do seem to involve it,“ explains Dr. Katy Moncivais, scientific advisor at ConsumerSafety.org. The organization provides legal support, with a special focus on medical issues.
Other known factors that influence the onset of mesothelioma are viral infections, genetic risk factors and radiation (Carbone et al., 2012). “In recent years, the percentage of cases without identifiable asbestos exposure has increased,” Moncivais says. “However, the academic community seems to lean toward a belief that many of these cases do involve asbestos exposure. But questions that would help to identify the source of exposure were not asked when recording the medical history of the patients.“


A broadly used substance:
The term asbestos refers to a group of silicate minerals formerly used as building in insulation across many industries.
Stopping malignant mesothelioma by triggering the immune system
One approach that makes use of this heterogeneity is developing a therapy that aims specifically at the tumor using the patient’s own immune system. “The immune system is so much more powerful and specific than our blunt treatments like chemo and radiation. They’re kind of like using a hammer to beat down a door – instead of just using the key to open it,“ says Moncivais. To turn a specific therapy into reality, T cells from a patient are isolated, transferred to a cell culture and engineered to specifically attack malignant cells (June et al., 2018). The approach is known as chimeric-antigen-receptor (CAR) T cell therapy. Identifying the molecular marker that the therapy is aiming at, is the key to this approach (Klampatsa et al., 2017).
A promising target could be mesothelin, a cell-surface glycoprotein that is overexpressed on malignant mesothelioma cells. A phase I clinical trial with 12 patients at the Memorial Sloan Kettering Cancer Center has shown initial success when giving each patient one dose of CAR-T cells that target the mesothelin of their mesothelioma. However, mesothelin is no magic bullet. “It’s not highly overexpressed in all mesothelioma subtypes. So, it may be a great target for some patients and a useless one in others,” says Moncivais. She notes that another promising target is fibroblast activating protein (FAP), a candidate for CAR-T cell therapy, and which is on the list of potential molecules published by a group of American researchers (Kiesgen et al., 2017). Even though the optimal molecular markers are still under investigation, Moncivais is quite sure about the most promising therapeutic approach for the future: “My money is definitely on CAR-T cell therapy.“
As more and more researchers investigate the opportunities of CAR-T cell therapy – the number of publications on PubMed has risen from 373 in 2008 to 858 in 2018 – large-scale production of those cells is becoming more important. Levine and his colleagues reviewed various approaches and challenges of manufacturing (Levine et al., 2016). They conclude that deep knowledge of processes and strict adherence to clinical manufacturing practices are extremely important when culturing cells of a patient in vitro and preparing them for reinfusion as a CAR-T cell therapeutic.

An expert in mesothelioma:
Katy Moncivais, PhD, has a background in bioengineering and regenerative medicine. She works as a scientific advisor at ConsumerSafety.org. Picture kindly provided by Dr. Katy Moncivais.
Using 3D cell culture delivers promising results
Re-establishing the in vivo conditions in vitro is essential for generating knowledge about malignant mesothelioma. This is the issue facing several cancer therapies. Scientists aim to find new active substances that are effective not only in 2D cell culture settings, but also in 3D experiments, which are closer to the conditions in the patient. Gwendolyn Cramer and her team of researchers addressed this issue by developing a 3D malignant pleural mesothelioma model (Cramer et al., 2018). They combined different cell lines with various approaches for generating 3D cultures. Using GFR-Matrigel, the researchers succeeded at growing 3D structures with different cell lines, which led to different phenotypes within the 3D structure. Finally, the researchers used the model to assess the effect of two combined therapeutic interventions, namely photodynamic therapy and EGFR-inhibition. The combination of both approaches led to an increased cytotoxicity, as confirmed by their findings.
Researchers around Andrea Mazzocchi went one step further by developing individual tumor organoids from two mesothelioma patients (Mazzocchi et al., 2018). They extracted the tumor cells from the obtained tissue and transferred them into a microfluidic device. Using their 3D tumor constructs, they screened several chemotherapeutics and identified a non-standard component to be effective for therapy due to a particular mutation of the patient. This platform technology could, of course, be extended to therapeutic options other than chemotherapy, as well as to other types of cancer.
A new weapon against cancer:
Triggering the patient’s immune system to fight malignant cells is a promising therapeutic approach that is gaining attention.
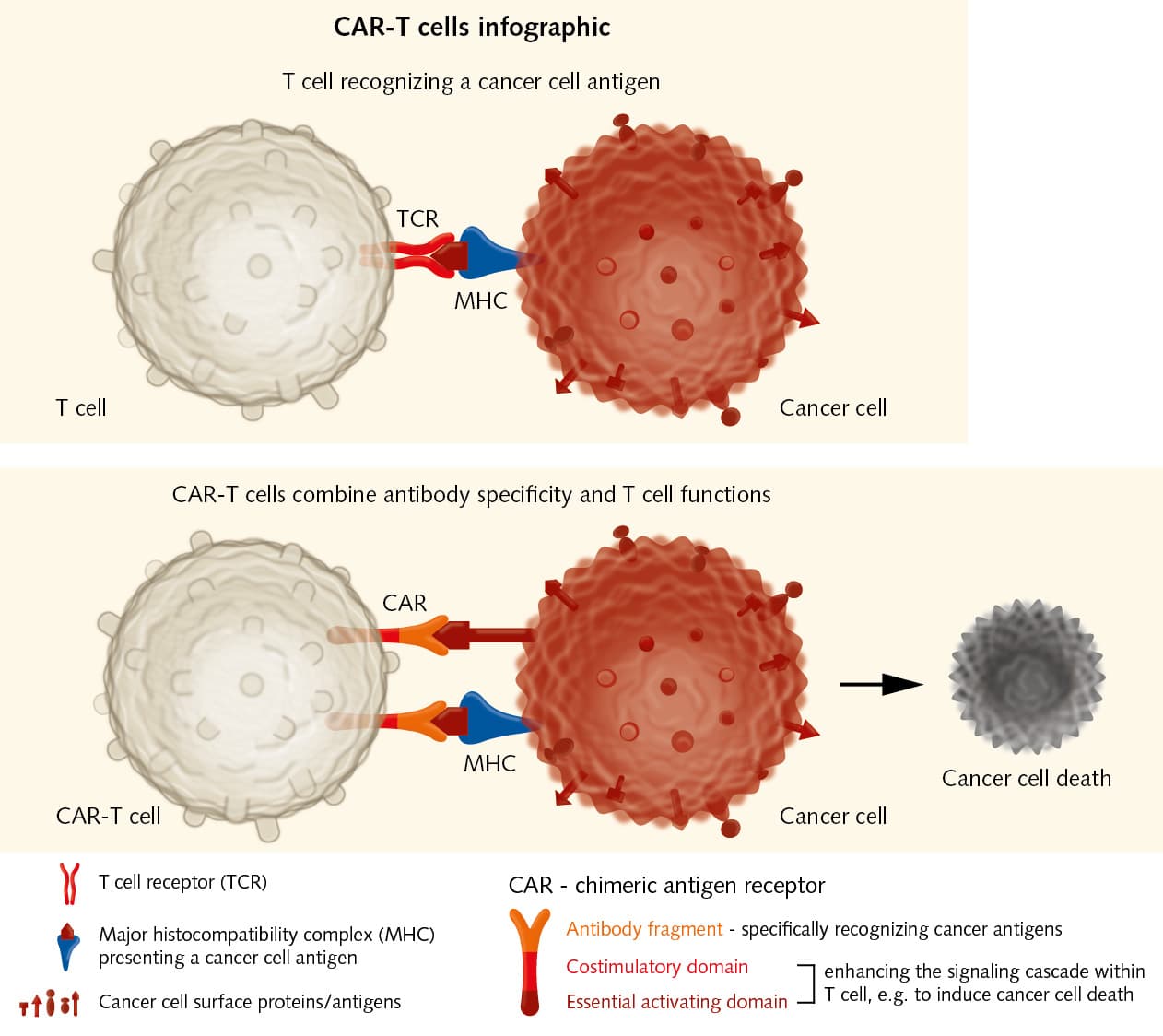
Adoptive transfer of effector T cells:
Chimeric Antigen Receptor immunotherapy has shown good results in the treatment of hematological malignancies. It could also be used for solid tumors. CAR-T cells combine recognition specificity of antibodies with T cell functions, e.g. cytotoxicity, and thereby enhance immune responses against cancer cells.
Upgrade Your Cancer Cell Culture
Isolate tumor cells from patient samples and transform them into long-term cell cultures.
Learn more about the Primary Cancer Culture System and how it compares to other established culture methods.
Related resources
Related pages


Cancer Cell Culture
Our cancer cell culture portfolio provides innovative solutions to establish primary cancer cell lines from biopsies, develop 3D tumor models or build screening assays. The serum-free and xeno-free formulation of our cancer cell culture media allow for an optimally adapted and highly standardized environment for 3D spheroids and 2D adherent monolayers.

3D Cell Culture
In their natural physical environment, cells that form tissues and organs do not exist as single cell entities but are surrounded by other cells and are part of larger multicellular organisms. They are embedded within a complex non-cellular structure known as the extracellular matrix (ECM) which forms an interface between the cells and their adjacent stroma – anchoring and agglutinating the cells in a three-dimensional (3D) formation.
