
3D Cell Culture
Find suitable products, scientific resources or answers to your questions within our technical library FAQ.
Checkout using your account
This form is protected by reCAPTCHA - the Google Privacy Policy and Terms of Service apply.
Checkout as a new customer
Creating an account has many benefits:
Cancer stem cells (CSCs) are a specific and small subpopulation of tumor cells. Despite their low number, they drive tumor initiation, influence tumor progression, and cause metastasis and relapse. Increased cancer stem cell populations have been shown to reflect the aggressiveness and therapy-resistant phenotype of the tumor.
Compounds targeting CSCs have demonstrated the potential to increase the efficacy of existing and experimental chemotherapeutic regimens in response to cancer cells resistant to chemotherapy. Therefore, the persistent availability and identification in the experimental setup of CSCs is important to understand tumor biology, identify cancer biomarkers, and develop effective cancer treatments.
When working with CSCs, researchers are typically confronted with the following challenges:
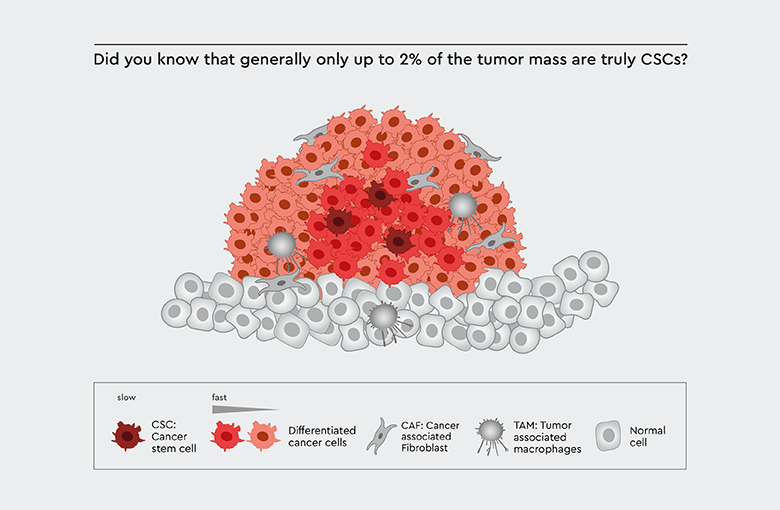

Figure 1: Tumor biology. Cancer stem cells (CSCs) play a pivotal role in tumor biology: The tumor mass is derived from progenitor cells of the CSCs.
The experimental design needs to ensure the proper cultivation of tumor-driving CSCs to maintain the tumor characteristics and tumorigenicity. Failure in the experimental set up will result in flawed results such as:
Ignoring CSCs is like trying to grow an apple tree without a stem or trunk. But how to bring the selection and cultivation of CSCs to the next level?
A simple, but yet effective and reliable method for selection and long-term cultivation of CSCs
The ideal solution for cultivating cancer stem cells is to isolate them from the starting material and to keep them in in vitro culture as long as needed for the experiment
We have developed a toolbox of different functional media, that (1) selects CSCs from any starting material, (2) establishes a primary cancer cell line and (3) keeps a defined and healthy CSC subpopulation in numerous experimental set ups such as tumorspheres, co-cultivation with other non-tumor cells, screening-assays, long-term studies and alike.
The media meet exactly the metabolic and nutritional requirements commonly shared by most cancer stem cells. Consequently, there is no need to initially or repetitively check for the presence of CSCs, cell sorting or re-sorting. All media support multiple-passaging and long-term cultivation up to frozen master stocks. Depending on the choice of a medium of this toolbox, it can be controlled, which other cell-types (cancer cells, cancer associated cells or non-cancer cells) shall be co-cultured. Especially the Primary Cancer Culture System (PCCS) is designed to obtain pure CSC populations.
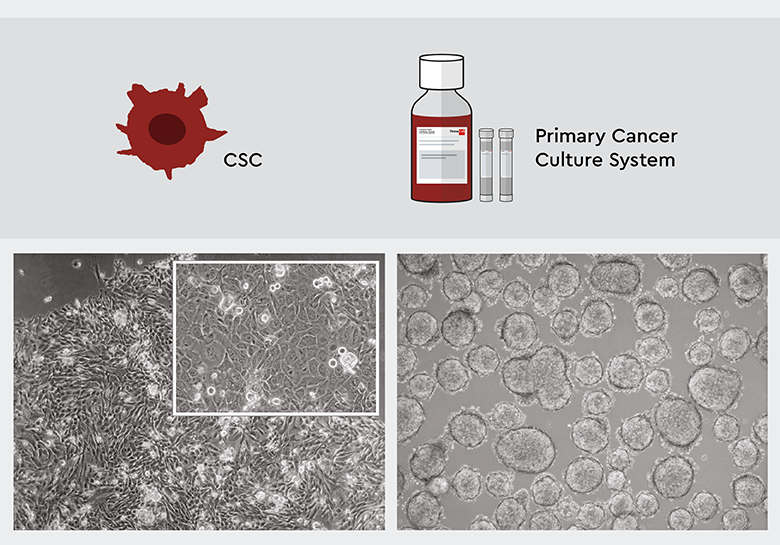

Figure 2: The PCCS selects and maintains specifically CSCs. Left: Four weeks after isolation, the primary culture derived from squamous cell carcinoma features an adherent growth pattern. The upper right inset shows that CSC heterogenity is maintained. Right: Primary culture derived from a low-grade from a low-grade small lung cancer after 4 weeks. The cell started 3D sphere formation indicating that tumor biology maintained.
There is yet another essential advantage of these media. They can be used for different cancer cell lines and tumors because CSCs share common metabolic properties across most cancer types, which are supported through the design of each medium. There is no need for medium optimization for individual cell lines and biopsy materials to support CSCs and hence the battery of media protocols is substantially reduced. The media have been tested in over 30 cell lines and tumor types internally as well as with customers.
Having media that support CSCs rather than individual cancer cell types – we call it universal media – avoids an artificial bias for cancer cells that grow best in a given standard or particular purpose medium or were selected based on non-common CSC biomarkers. Instead, our toolbox supports the spectrum of CSCs subpopulations and early progenitors.
Learn more about the Principle of our Primary Cancer Culture System (PCCS)
Workflow for the effective and reliable selection and long-term cultivation of CSCs
Using the PCCS is an efficient and convenient way to select cancer stem cells. The principal workflow using PCCS is shown in Figure 3:
Note: in contrast to fast growing malignant cancer cells, stem cells exhibit naturally a slower growth and proliferation pattern


Figure 3: Principal workflow for cancer stem cell selection with the PCCS starting with established cell lines. Selecting CSC using our PCCS makes frequent control for the presence of CSCs by repetitive re-sorting steps obsolete.
Workflow for a simple selection and long-term cultivation of CSCs for 3D culture
The PCCS can also be used to select cancer stem cells from biopsies. In contrast to the procedure for selecting CSCs from established cell lines, biopsies contain many different cancer cells, cancer-associated cells and non-cancer cells. The principal workflow using PCCS is shown in Figure 4:
Note: this workflow is focused on obtaining CSCs and early progenitors, which should be further investigated. If the experimental purpose requires non-cancer cell types, then check out our 3D Cancer Model


Figure 4: Principal workflow for cancer stem cell selection with the Primary Cancer Culture System (PCCS) or 3D Tumorsphere Medium XF starting with biopsy material. Depending on the malignancy of the tumor biopsy, primary 2D and 3D cultures can be established.
Why is the PCCS able to isolate CSCs from various starting materials?
We have taken a different approach for the development of CSCs supporting media. Rather than designing a special purpose medium for each cancer and tissue type, we designed a media technology that support CSCs independently from their origin. CSCs are capable to migrate throughout the body and hence can survive in different organ and tissue environments. This is because of the CSCs’ self-renewal capability, Anoikis resistance, the transition between a state of quiescence and proliferation, but also a different and aberrant metabolism. The latter property is addressed in the design of the toolbox.
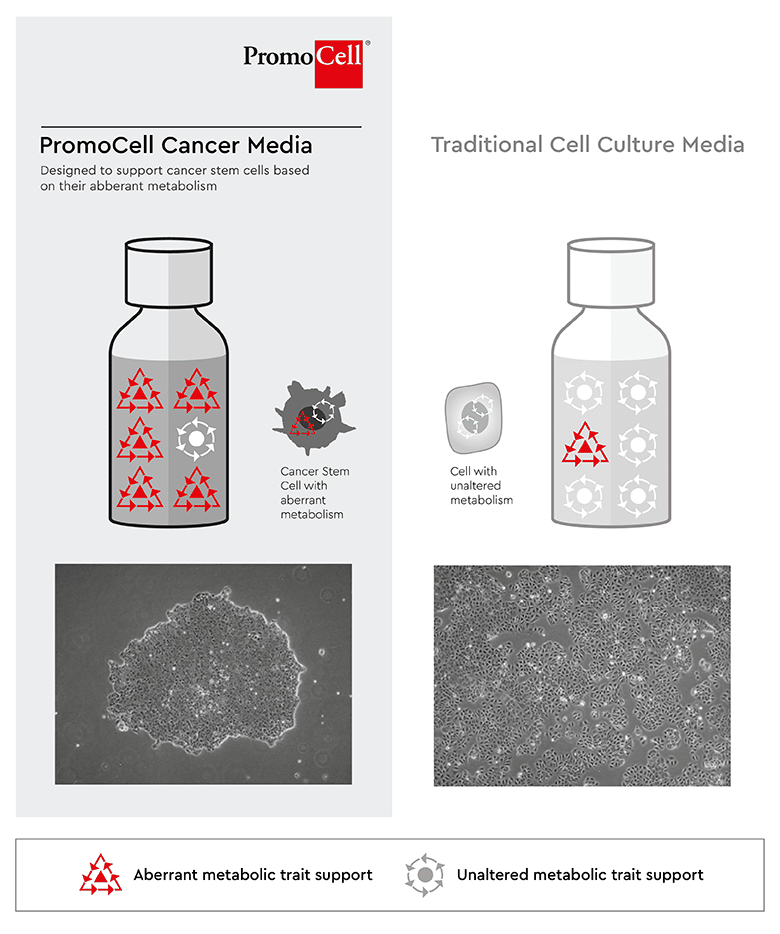

Figure 5: Mechanism of the PCCS. Left: Selection of CSCs by culturing cancer cell lines in the PCCS leads to compact colonies consisting of CSCs with a remarkably homogenous appearance. Right: The original culture in the standard medium consists of larger cells and shows a heterogeneous morphology.
The functional media of the toolbox meet exactly the requirements commonly shared by most cancer stem cells and solve the two critical problems:
To make it a true toolbox, the media are designed to either only support CSCs or to support CSCs together with other cell types.
Isolation of CSCs from biopsy
Isolation of CSC s from tumor biopsies is critical for the development of new cancer therapies targeting treatment resistance, tumor recurrence, or cancer metastasis.
Our PCCS supports the isolation of cancer stem cells from liver metastases of gastrointestinal stromal tumors (GIST).
Cancer stem cell isolation from GIST liver metastasis
Using our PCCS, CSCs can be isolated and cultivated from resected liver metastases of GIST according to the protocol in our Application Note Isolation of patient-derived primary cancer cells from GIST liver metastasis.
After homogenization and enzymatic digestion of the tumor tissue, the cells are plated on NCCD -treated tissue culture vessels and allowed to grow for 13 days before passaging. As shown in Figure 6, after 14 days of cultivation (i.e., day 1 after passage), the tumor-derived CSCs switch from suspension growth to adherent growth and appear as proliferating spindle-shaped cells, a morphology typical of stromal tumor-derived cells.


Figure 6: CSC isolation from GIST liver metastasis using PCCS. Left: Floating multicellular cell aggregates on day 4 (passage 0) after isolation and initial plating (40x magnification). Center: On day 14 (passage 1) after isolation using Primary Cancer Cell Medium D-ACF, primary GIST cells appear as adherently growing cell clusters. Right: After 17 days (passage 1) of cultivation, GIST-derived cancer cells proliferate and exhibit a spindle-shaped morphology (100x magnification).
CSCs isolated from liver metastases form a nearly confluent 2D monolayer 49 days after isolation and cultivation (Figure 7).


Figure 7: CSC long-term expansion in PCCS (C-28081). CSC proliferation GIST-derived CSCs from liver metastasis 49 days after isolation (passage 2). CSCs maintain a spindle-shaped cell morphology and form a 2D monolayer (100x magnification).
CSCs isolated from GIST liver metastasis in passage 3 were subjected to immunostaining for the transcription factors and stem cell markers SOX2 and Oct4. Double-stained cells were analyzed using flow cytometry.


Figure 8: Flow cytometric analysis of CSCs isolated from GIST liver metastases. CSCs were isolated from liver metastases and cultivated until passage 3 using the PCCS. Cells were double-stained using antibodies specific for the stem cell markers Oct4 and SOX2. Flow cytometric analysis revealed that 97.5% of the cells were positive for both markers, indicating a homogenous cell population with stem cell properties.
Oct4, also known as POU5F1, belongs to a family of transcription factors that regulate stem cell pluripotency. SOX2 is a stem cell marker that regulates organ development. In CSCs, the functional interaction of SOX2 and Oct4 regulates various stem cell characteristics. Therefore, the co-expression of Oct4 and SOX2 represents an excellent and reliable marker of stem cells.
Next-generation sequencing analysis revealed the presence of a heterozygous mutation in the KIT proto-oncogene that is commonly found in GIST. The KIT gene encodes a receptor tyrosine kinase, the mutation of which is associated with GIST. The heterozygous mutation in KIT occurred in 98% of the analyzed cells in passage 3.
Have a look at other Research Areas
PromoCell uses HubSpot to provide LiveChat support. This feature is currently blocked due to your cookie preferences.
Accept cookies to enable LiveChat