Imagine a future where diseases once considered incurable are now within reach of treatment. This is the promise of stem cell immunotherapy, a rapidly evolving approach that harnesses the unique properties of pluripotent cells to revolutionize medical treatments.1 From autoimmune diseases to cancer, the potential of stem cell immunotherapy is vast and transformative. In this article, we explore the principles of stem cell immunotherapy, its potential applications, and future possibilities.
How does stem cell immunotherapy work?
The immune system defends the body against pathogens and tumors. However, when immune responses become dysregulated, they can drive diseases like autoimmunity and chronic inflammation, as well as transplant rejection.3 Stem cell immunotherapy aims to correct aberrant immunity by leveraging the unique immunomodulatory properties of stem cells, which can be obtained from various sources, including bone marrow, adipose tissue, and umbilical cord blood.4 Stem cells are undifferentiated cells that can differentiate into specialized cells, including immune cells, and can be used to replace or repair damaged tissues.5 Stem cell immunotherapy involves the administration of multipotent cells with immunomodulatory properties, such as mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs).1,2 These cells can migrate to sites of inflammation and injury and modulate pathogenic immune responses by interacting with various immune cell types and secreting factors that can dampen inflammation and promote tolerance.1,2 In addition to their immunomodulatory properties, they have a remarkable ability for self-renewal and differentiation, providing additional advantages for their use to promote tissue repair and regeneration.6 Stem cells can:- Suppress overactive immune responses by decreasing the production of inflammatory cytokines and inducing the secretion of anti-inflammatory cytokines.7
- Promote regulatory T cell responses and tolerogenic dendritic cell responses to induce immunological tolerance.8
- Enhance tissue repair in damaged tissues by secreting growth factors and stimulating regeneration and repair.9
Potential applications of stem cell immunotherapy
Stem cell immunotherapy is being tested for various conditions in which modulating immunity could prove beneficial. This emerging field holds great promise for transforming medicine and represents an exciting new frontier in stem cell research and regenerative medicine. By leveraging the immunomodulatory effects of stem cells, this innovative approach opens new avenues for the treatment of a wide range of conditions, including autoimmunity, transplant rejection, and cancer.2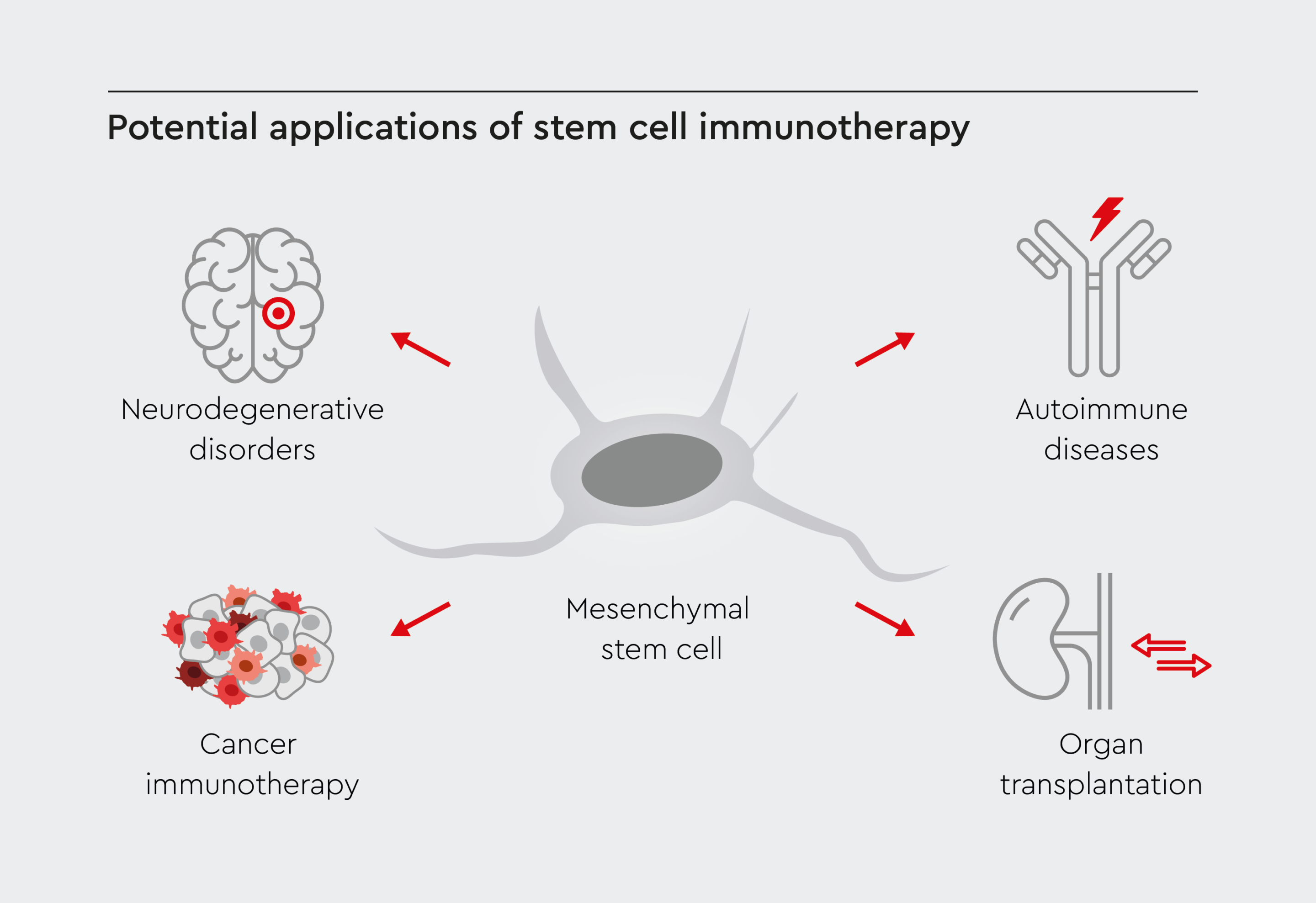
Figure 1: Potential stem cell-based immunotherapy applications.
Stem cell immunotherapy has the potential to be used to treat a wide range of diseases and disorders, addressing a variety of conditions such as cancer or neurological dysfunction.
Autoimmune diseases
In autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and type 1 diabetes, the immune system mistakenly attacks the body's own tissues. Cell-based therapies may restore immune balance and promote self-tolerance in these disorders.10 For example, hematopoietic stem cell transplantation (HSCT), involving the harvesting and reintroduction of stem cells after high-dose chemotherapy, can be used to rebuild a properly functioning immune system.11 Another approach is to selectively suppress the immune response by using stem cells. For instance, stem cells with immunomodulatory properties, such as MSCs, could be used to suppress the immune system's overactive response in patients with rheumatoid arthritis, alleviating inflammation in the joints and potentially slowing the progression of the disease.12 In the case of inflammatory bowel disease, which includes conditions such as Crohn's disease and ulcerative colitis, excessive inflammation in the gastrointestinal tract causes significant discomfort and damage. Stem cell immunotherapy could help modulate the immune response, reducing inflammation and allowing the gut to heal.13Organ transplantation
Stem cells could reduce transplant rejection by dampening recipient anti-graft immune responses.14 Therefore, stem cell immunotherapy could reduce the risk of rejection and enhance the success of these life-saving procedures. Moreover, infusions of immunomodulatory stem cells allow tapering of immunosuppressive drugs that leave transplant patients vulnerable to infections.14Neurodegenerative disorders
Neuroinflammation drives progression in neurodegenerative diseases, such as Alzheimer's and Parkinson's disease.15 Stem cells may protect vulnerable neurons from neurotoxic inflammation and stimulate neuroregeneration by secreting anti-inflammatory cytokines and growth factors.16 In Parkinson's disease, they can replace damaged or lost dopaminergic neurons, potentially alleviating motor symptoms and improving patients' quality of life.17 Similarly, in the context of spinal cord injuries, stem cell immunotherapy aims to restore neural connections and promote functional recovery, offering hope to those affected by debilitating trauma.18Cancer immunotherapy
Cancer hijacks the body's defense mechanisms, thereby inhibiting anti-tumor immunity.19 Researchers are exploring the use of stem cells to boost the immune system's ability to fight cancer cells.20 In addition, combining immune checkpoint inhibitors or chimeric antigen receptor (CAR)-T cell therapy with stem cell immunotherapy could synergistically activate anti-cancer immunity.21 HSCT is often used to reset the immune system in patients with hematologic malignancies, such as leukemia, multiple myeloma, and lymphoma.22 Moreover, stem cells can be genetically engineered to express specific anti-cancer molecules, empowering the immune system to identify and eliminate cancer cells more effectively.23Advantages of stem cell immunotherapy
Stem cell-based immunotherapy provides several benefits over traditional pharmacological treatments:- Targeted: Stem cells migrate to sites of inflammation or tissue damage, providing localized immune modulation where needed.24 This means that stem cell immunotherapy could have fewer side effects than conventional pharmacotherapies.
- Multimodal: Unlike many drugs, stem cells simultaneously act through multiple complementary mechanisms to restore immune balance.25
- Responsive: They can sense microenvironmental cues and respond by adapting their function.26
- Regenerative: They can replace damaged cells and stimulate tissue regeneration.9
- Long-lasting: Unlike conventional drugs that require repeated dosing, these cells have self-renewal ability. Therefore, one or a few rounds of treatment could potentially provide long-lasting benefits.5
- Versatile: They can differentiate into various cell types, offering versatility in treating different diseases.5
Challenges and ethical considerations
Although stem cell immunotherapy shows promise in the treatment of various diseases, there are also challenges and safety concerns that warrant careful consideration:- Safety: Culture-expanded pluripotent cells can undergo mutations or have altered functions.27 Therefore, rigorous quality control is required to ensure their safety and purity.
- High costs: Treatments can be expensive, making them less accessible to many patients and raising questions about affordability and insurance coverage.28
- Immunogenicity: Recipient anti-donor immune responses may clear allogeneic cells.29 Strategies to reduce immunogenicity are needed.
- Maximizing potency: More research on optimizing stem cell manufacturing, dosing, route of administration, and combination strategies will help improve efficacy.30
- Ethical concerns: Embryonic cell research faces various ethical challenges.31 Induced pluripotent stem cells from adult tissues provide an alternative strategy to overcome the ethical challenges of using embryonic cells.
- Regulation: Regenerative medicine regulations continue to evolve to ensure the safety, efficacy, and ethical sourcing of cell products.31 The lack of standardized regulatory guidelines may lead to variations in quality control, potentially compromising the safety and efficacy of treatments.
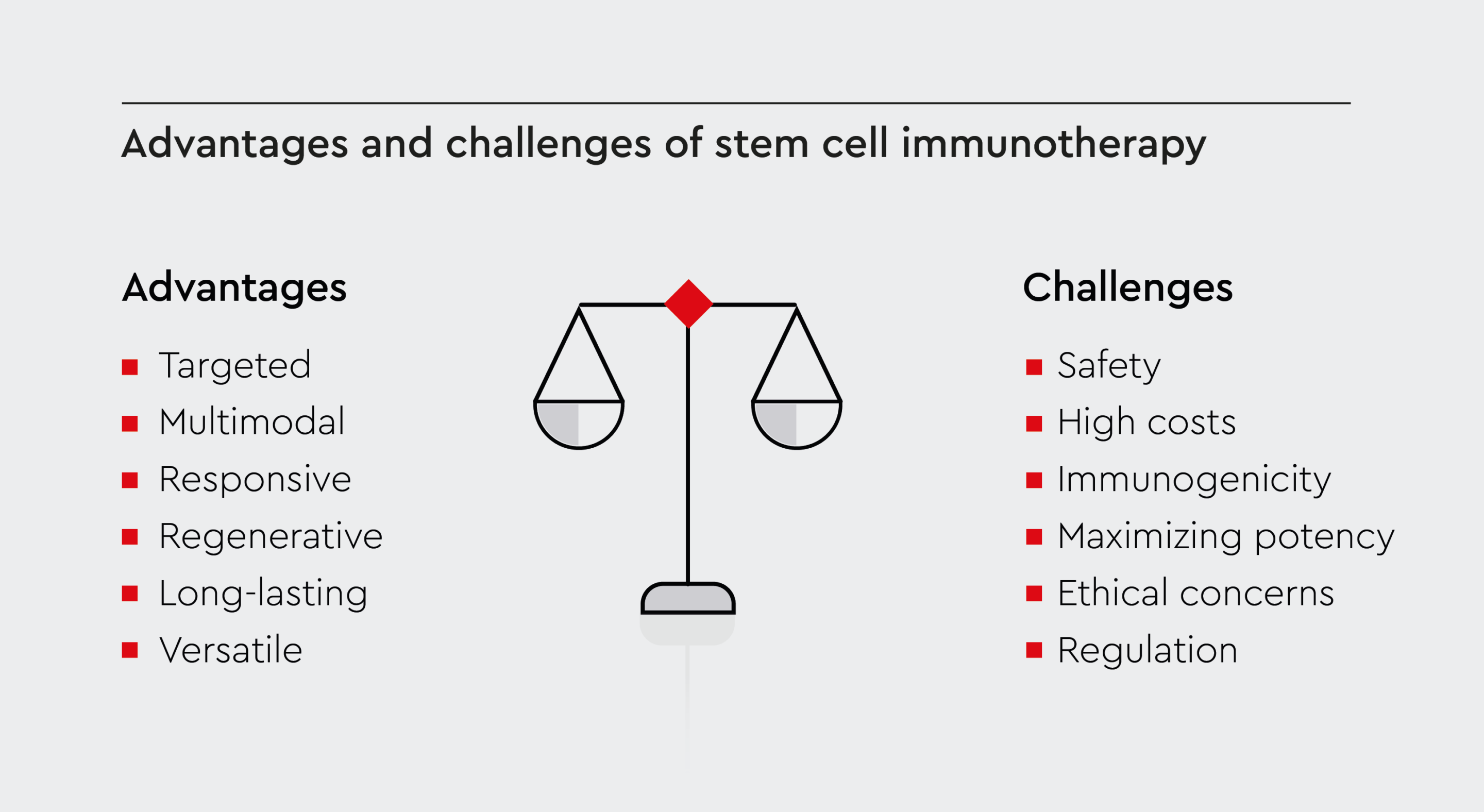
Figure 2: Stem cell therapy vs. conventional treatment.
Key aspects of stem cell-based immunotherapy, including benefits over traditional treatments and challenges, such as high costs, regulatory or ethical concerns in stem cell research.
The future of stem cell immunotherapy
Cell-based immunotherapies have shown promising results in the treatment of several diseases. For example, CAR-T cell therapy has been used to treat leukemia, lymphoma, and multiple myeloma.32 Stem cell immunotherapies are currently in clinical trials for various diseases, including cancer, autoimmune diseases (e.g., multiple sclerosis and rheumatoid arthritis), and neurodegenerative disorders.20,33,34 These trials aim to evaluate the safety and efficacy of stem cell-based therapies and identify the optimal dose and route of administration. From refining techniques in immune modulation to exploring novel applications in age-related diseases, the field is constantly evolving. As research advances, therapies using pluripotent cells may one day provide:- Personalized medicine: Patient-derived induced pluripotent stem cells (iPSCs) ensure immunocompatibility and suitability for personalized therapies.35
- Combination strategies: Combining stem cells with immunomodulatory drugs (such as JAK inhibitors) or biologics (such as recombinant cytokines) may provide synergistic benefits.36
- Tissue engineering: Combining 3D-printing and stem cells could enable lab-grown replacement organs resistant to rejection.37
- In vivo reprogramming: Converting a patient's cells into stem cells within the body could stimulate tissue repair while avoiding technical challenges with sourcing these cells and ethical considerations of using embryonic cells.38
References
Expand
- Anderson JD. Advances in Stem Cell Immunotherapy. Stem Cells. 2023;41(4):307-309. doi:10.1093/stmcls/sxad011
- Marcuzzi A, Maximova N. Editorial: Advances in stem cell therapy: new applications and innovative therapeutic approaches. Front Med. 2023;10:1225551. doi:10.3389/fmed.2023.1225551
- Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S3-23. doi:10.1016/j.jaci.2009.12.980
- Wada N, Gronthos S, Bartold PM. Immunomodulatory effects of stem cells. Periodontol 2000. 2013;63(1):198-216. doi:10.1111/prd.12024
- Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. doi:10.1186/s13287-019-1165-5
- Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem cell Investig. 2019;6:19. doi:10.21037/sci.2019.06.04
- Kyurkchiev D, Bochev I, Ivanova-Todorova E, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6(5):552-570. doi:10.4252/wjsc.v6.i5.552
- Ness S, Lin S, Gordon JR. Regulatory dendritic cells, T cell tolerance, and dendritic cell therapy for immunologic disease. Front Immunol. 2021;12:633436. doi:10.3389/fimmu.2021.633436
- Jin Y, Li S, Yu Q, Chen T, Liu D. Application of stem cells in regeneration medicine. MedComm. 2023;4(4):e291. doi:10.1002/mco2.291
- Ghobadinezhad F, Ebrahimi N, Mozaffari F, et al. The emerging role of regulatory cell-based therapy in autoimmune disease. Front Immunol. 2022;13:1075813. doi:10.3389/fimmu.2022.1075813
- Snowden JA, Sharrack B, Akil M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases - a guide for the generalist. Clin Med. 2018;18(4):329-334. doi:10.7861/clinmedicine.18-4-329
- Sarsenova M, Issabekova A, Abisheva S, Rutskaya-Moroshan K, Ogay V, Saparov A. Mesenchymal stem cell-based therapy for rheumatoid arthritis. Int J Mol Sci. 2021;22(21). doi:10.3390/ijms222111592
- Zhang H-M, Yuan S, Meng H, et al. Stem cell-based therapies for inflammatory bowel disease. Int J Mol Sci. 2022;23(15). doi:10.3390/ijms23158494
- Deo D, Marchioni M, Rao P. Mesenchymal stem/stromal cells in organ transplantation. Pharmaceutics. 2022;14(4). doi:10.3390/pharmaceutics14040791
- Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023;8(1). doi:10.1038/s41392-023-01486-5
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5(6):933-946. doi:10.2217/rme.10.72
- Zeng X, Qin H. Stem cell transplantation for Parkinson's disease: current challenges and perspectives. Aging Dis. 2022;13(6):1652-1663. doi:10.14336/AD.2022.0312
- Zeng C-W. Advancing spinal cord injury treatment through stem cell therapy: A comprehensive review of cell Types, challenges, and emerging technologies in regenerative medicine. Int J Mol Sci. 2023;24(18). doi:10.3390/ijms241814349
- Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915-919. doi:10.1158/2159-8290.CD-15-0563
- Chu D-T, Nguyen TT, Tien NLB, et al. Recent progress of stem cell therapy in cancer treatment: molecular mechanisms and potential applications. Cells. 2020;9(3). doi:10.3390/cells9030563
- Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471-482. doi:10.1016/j.ccell.2019.09.006
- Patel SA, Rameshwar P. Stem cell transplantation for hematological malignancies: prospects for personalized medicine and co-therapy with mesenchymal stem cells. Curr Pharmacogenomics Person Med. 2011;9(3):229-239. doi:10.2174/187569211796957548
- Li Y-R, Dunn ZS, Yu Y, Li M, Wang P, Yang L. Advancing cell-based cancer immunotherapy through stem cell engineering. Cell Stem Cell. 2023;30(5):592-610. doi:10.1016/j.stem.2023.02.009
- Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33(3):136-143. doi:10.1016/j.it.2011.11.004
- Aurora AB, Olson EN. Immune modulation of stem cells and regeneration. Cell Stem Cell. 2014;15(1):14-25. doi:10.1016/j.stem.2014.06.009
- Keung AJ, Kumar S, Schaffer D V. Presentation counts: microenvironmental regulation of stem cells by biophysical and material cues. Annu Rev Cell Dev Biol. 2010;26:533-556. doi:10.1146/annurev-cellbio-100109-104042
- Wang Y, Zhang Z, Chi Y, et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013;4(12):e950. doi:10.1038/cddis.2013.480
- Dresser R. Stem cell research as innovation: expanding the ethical and policy conversation. J law, Med ethics a J Am Soc Law, Med Ethics. 2010;38(2):332-341. doi:10.1111/j.1748-720X.2010.00492.x
- Lohan P, Treacy O, Griffin MD, Ritter T, Ryan AE. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: are we still learning? Front Immunol. 2017;8:1626. doi:10.3389/fimmu.2017.01626
- Liu S, Zhou J, Zhang X, et al. Strategies to optimize adult stem cell therapy for tissue regeneration. Int J Mol Sci. 2016;17(6). doi:10.3390/ijms17060982
- Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30(3):204-213. doi:10.1210/er.2008-0031
- Wang Z, Chen C, Wang L, Jia Y, Qin Y. Chimeric antigen receptor T-cell therapy for multiple myeloma. Front Immunol. 2022;13:1050522. doi:10.3389/fimmu.2022.1050522
- Mohammedsaleh ZM. The use of patient-specific stem cells in different autoimmune diseases. Saudi J Biol Sci. 2022;29(5):3338-3346. doi:10.1016/j.sjbs.2022.02.009
- Sivandzade F, Cucullo L. Regenerative stem cell therapy for neurodegenerative diseases: an overview. Int J Mol Sci. 2021;22(4). doi:10.3390/ijms22042153
- Paik DT, Chandy M, Wu JC. Patient and disease-specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacol Rev. 2020;72(1):320-342. doi:10.1124/pr.116.013003
- Sarsenova M, Kim Y, Raziyeva K, Kazybay B, Ogay V, Saparov A. Recent advances to enhance the immunomodulatory potential of mesenchymal stem cells. Front Immunol. 2022;13:1010399. doi:10.3389/fimmu.2022.1010399
- Shinkar K, Rhode K. Could 3D extrusion bioprinting serve to be a real alternative to organ transplantation in the future? Ann 3D Print Med. 2022;7:100066. doi:https://doi.org/10.1016/j.stlm.2022.100066
- Romanazzo S, Lin K, Srivastava P, Kilian KA. Targeting cell plasticity for regeneration: From in vitro to in vivo reprogramming. Adv Drug Deliv Rev. 2020;161-162:124-144. doi:https://doi.org/10.1016/j.addr.2020.08.007
Contact our experts
If you're a researcher or clinical scientist striving to develop stem cell treatments, contact our specialists to learn more about how our products can advance your research and move stem cell immunotherapy closer to the clinic.
Contact us