The use of mesenchymal stem cells in regenerative medicine is a scientific success story that started approximately 40 years ago. Although mesenchymal stem cells were initially met with skepticism, they now form the foundation of thousands of research studies and hundreds of clinical trials. In this article, we discuss how the origin of mesenchymal stem cells may impact their properties and therapeutic potential.
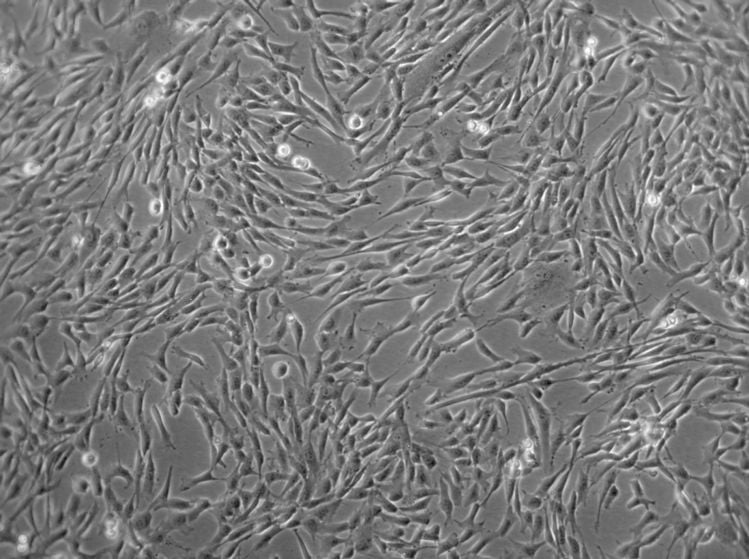

Figure 1: Microscopic image of cultured human mesenchymal stem cells. MSCs typically exhibit elongated, spindle-shaped morphology when cultured in vitro.
Evolution of mesenchymal stem cell research
It can take time until a revolutionary idea takes hold. This means that pioneers with progressive ideas often receive no recognition during their lifetimes.1,2 This was the case for the godfathers of experimental hematology, Alexander Maximow (1874–1928) and Alexander Friedenstein (1924–1998).
These visionaries laid the experimental groundwork for what would become a cornerstone of regenerative medicine and cell-based therapies. Friedenstein, now credited with discovering mesenchymal stem cells (MSCs), built upon Maximow’s initial research despite facing considerable skepticism from the scientific community.1,2
Despite facing significant skepticism, the work of Maximow and Friedenstein has stood the test of time. Today, thousands of publications focusing on MSCs can be found in the PubMed database from the US National Library of Medicine at the National Institutes of Health. In 2024 alone, more than 9000 publications related to MSCs were added to the scientific database. This exponential growth reflects the increasing recognition of the therapeutic potential of MSCs across various clinical applications.
Understanding the origins of mesenchymal stem cells
MSCs are adult stem cells believed to originate from perivascular cells harbored in blood vessel walls.3,4 Dr. Hagen Wieland, who has been working with MSCs for more than 15 years and leads the Research and Development team at PromoCell, explained that these multipotent progenitor cells can be found in virtually every tissue type and that their abundance correlates with blood vessel density. Their primary function involves maintaining tissue homeostasis — a vital process for proper body function.
The versatility of MSCs stems from their ability to differentiate into various cell types, including:
- Mesodermal lineage, which gives rise to osteocytes, adipocytes, chondrocytes, and myocytes.5
- Ectodermal lineage, which generates neurons.5
- Endodermal lineage, which produces hepatocytes and insulin-producing β-cells.5


Dr. Hagen Wieland
Team Leader – Research and Development at PromoCell
Understanding the origins of mesenchymal stem cells
MSCs are adult stem cells believed to originate from perivascular cells harbored in blood vessel walls.3,4 Dr. Hagen Wieland, who has been working with MSCs for more than 15 years and leads the Development Department at PromoCell, explained that these multipotent progenitor cells can be found in virtually every tissue type and that their abundance correlates with blood vessel density. Their primary function involves maintaining tissue homeostasis — a vital process for proper body function.
The versatility of MSCs stems from their ability to differentiate into various cell types, including:
- Mesodermal lineage, which gives rise to osteocytes, adipocytes, chondrocytes, and myocytes.5
- Ectodermal lineage, which generates neurons.5
- Endodermal lineage, which produces hepatocytes and insulin-producing β-cells.5


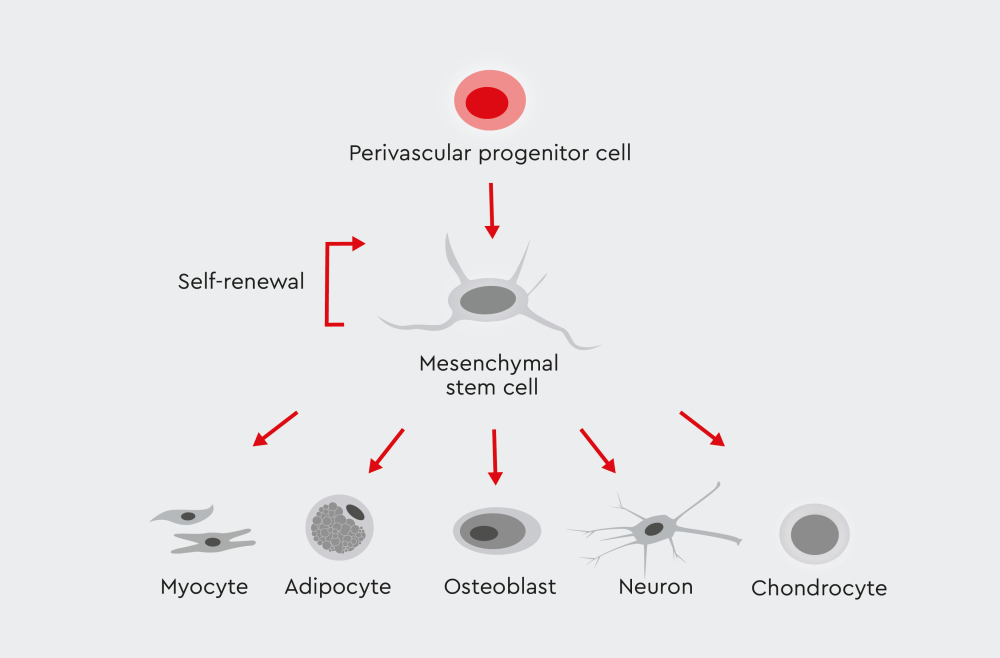

Figure 1: Multipotency of mesenchymal stem cells. These adult stem cells can be derived from numerous tissues and organs. They show the ability to differentiate into various cell types, including monocytes, adipocytes, osteoblasts, neurons, and chondrocytes.
Tissue-specific differences in MSC populations
What are tissue-specific MSCs? Recent research has revealed that the origin of mesenchymal stem cells significantly influences their characteristics and therapeutic potential. Different tissue sources yield MSC populations with distinct properties:
- Bone marrow-derived MSCs are often considered the prototype.
- Adipose-derived MSCs may express CD34, contradicting traditional MSC criteria.
- Other tissue sources, such as the umbilical cord, dental pulp, and placenta, each produce MSCs with unique properties.
How do MSCs from different tissues differ in function? When we isolate MSCs from different origins, distinct stem cell populations may function differently and exhibit varying differentiation potential, Dr. Wieland explained. As an example, cells from adipose tissue can be CD34-positive, in contrast to the International Society for Cellular Therapy (ISCT) criteria positing that MSCs do not express CD34.6 Indeed, increasing evidence suggests that the expression of CD34 in MSCs depends upon cell origin, culture conditions, and passage number.7,8


Figure 2: Minimal criteria for mesenchymal stem cells. The ISCT has established specific markers and characteristics that differentiate MSCs from other cell types.
Dr. Wieland emphasized that differences in MSC populations are not merely academic — they have profound implications for clinical applications. The specific origin of MSCs may determine their efficacy for particular therapeutic uses. This variability also highlights the importance of using standardized protocols when isolating and characterizing MSCs derived from different tissues.
Importance of standardization in MSC research
Despite four decades of MSC research, standardization remains a significant challenge. According to Dr. Wieland, the isolation method and growth medium play crucial roles in obtaining well-defined MSC populations. However, many laboratories still use cell culture media that contain animal serum, the quality of which differs between batches, contributing to inconsistencies in research findings.
To overcome these challenges, researchers increasingly turn to:
- Defined and serum-free media
- Standardized isolation protocols
- Comprehensive cell characterization
Dr. Wieland acknowledged that many scientists are discouraged by the pricing of well-characterized MSCs and high-quality, defined media. He noted, however, that considering the long time required for troubleshooting, testing, and repeating experiments to achieve reproducible findings, the investment is worth it.
Only with detailed documentation of the origin of mesenchymal stem cells, methods, reagents, and subsequent characterization can experimental results be reliably compared across different laboratories. This standardization is becoming increasingly important as many scientific journals now require authentication of MSCs before accepting papers for publication.
Characterization of mesenchymal stem cells
Interested in learning more about how standardized characterization of MSCs can improve culture performance and research progress?


Figure 3: Fighting graft-versus-host disease.
During his PhD research, Alexander Hackel and his team at Essen University Hospital investigated the therapeutic potential of different MSC types. Picture kindly provided by Alexander Hackel.
MSCs: Applications in biomedicine
Dr. Alexander Hackel, now project leader at the University Hospital Schleswig-Holstein, is well aware of the heterogeneity of MSCs depending on their tissue origin, which can impact their therapeutic potential. During his PhD research at Essen University Hospital in Germany, Dr. Hackel investigated the effects of different MSC types on natural killer cells, T cells, and neutrophils.9,10 His team has also investigated the therapeutic benefits of MSCs in autoimmune and inflammatory diseases, such as graft-versus-host disease.11
The exponential growth of clinical trials is more than just a numerical trend, it reflects the increasing translational interest in MSCs. Originally studied for their role in tissue regeneration, MSCs are now recognized for their immunomodulatory, anti-inflammatory, and trophic functions, having led to hundreds of ongoing and completed clinical trials.
A major driver of this surge is the use of machine learning and AI, alongside innovative MSC priming strategies that enhance therapeutic efficiency, boost anti-inflammatory properties, reduce functional heterogeneity, and offer a safer, effective alternative to genetic modification. 10, 11,12
MSCs are being studied for their potential to:
- Prevent graft-versus-host disease (GvHD) in cases of resistance to steroid-based immunosuppressive therapies.13
- Treat immune disorders such as Crohn’s disease, multiple sclerosis, and neurodegenerative disorders.13
- Aid tissue repair following myocardial infarction.15
- Regenerate periodontal tissue defects.16
- Support hematopoietic stem cell transplantation.17
- Cartilage and bone regeneration.18
- Healthy aging and preventing chronic disease associated with aging.19
The future of tissue-specific MSC research
According to Dr. Wieland, the potential applications of MSCs are very broad. However, researchers need to fully understand how the origin of mesenchymal stem cells affects their function to be able to establish more targeted clinical applications based on their tissue-specific properties. The heterogeneity of MSCs, once seen as a challenge, is now recognized as an opportunity to customize treatments based on the tissue-specific properties of different MSC populations.
The establishment of standardized protocols for isolating, characterizing, and culturing MSCs from various tissue sources will accelerate research progress and clinical translation. This standardization will ensure that the therapeutic potential of these remarkable cells can be fully realized. Dr. Wieland is optimistic about the clinical applications of MSCs, noting that the time of MSCs has just begun!
References
Expand
- Afanasyev B, Elstner E, Zander A. A. J. Friedenstein, founder of the mesenchymal stem cell concept. Cellular Therapy and Transplantation. 2009;1:35–38. doi: 10.3205/ctt-2009-en-000029.01
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003
- Oh M, Nör JE. The perivascular niche and self-renewal of stem cells. Front Physiol. 2015;6. doi: 10.3389/fphys.2015.00367
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells – current trends and future prospective. Bioscience Reports. 2015;35(2):e00191. doi: 10.1042/BSR20150025
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905
- Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14(10):1159–1163. doi: 10.3109/14653249.2012.729817
- Viswanathan C, Kulkarni R, Bopardikar A, Ramdasi S. Significance of CD34 negative hematopoietic stem cells and CD34 positive mesenchymal stem cells. CSCR. 2017;12(6). doi: 10.2174/1574888X12666170502095625
- Petri RM, Hackel A, Hahnel K, et al. Activated tissue-resident mesenchymal stromal cells regulate NK cell function. Stem Cell Reports. 2017;9(3):985–998. doi: 10.1016/j.stemcr.2017.06.020
- Hackel A, Aksamit A, Bruderek K, et al. TNF‐α and IL‐1β sensitize human MSCs for IFN‐γ signaling. Eur J Immunol. 2021;51(2):319–330. doi: 10.1002/eji.201948336
- Hackel A, Vollmer S, Bruderek K, et al. Immunological priming of MSCs augments therapeutic benefits in GVHD. Front Immunol. 2023;14:1078551. doi: 10.3389/fimmu.2023.1078551
- Silva-Sousa T, Usuda JN, Al-Arawe N, et al. Systems biology and AI in stem cell research. Stem Cells. 2024;42(11):929–944. doi: 10.1093/stmcls/sxae054
- Ringdén O, Gustafsson B, Sadeghi B. MSCs in pediatric hematopoietic transplantation. Front Immunol. 2020;11:567210. doi: 10.3389/fimmu.2020.567210
- Biglari N, Mehdizadeh A, et al. MSCs in neurodegenerative disorders. Pathol Res Pract. 2023;247:154541. doi: 10.1016/j.prp.2023.154541
- Barrère-Lemaire S, Vincent A, et al. MSCs for cardiac function post-MI. Physiol Rev. 2024;104(2):659–725. doi: 10.1152/physrev.00009.2023
- Ouchi T, Nakagawa T. MSC-based therapies for periodontitis. Regenerative Therapy. 2020;14:72–78. doi: 10.1016/j.reth.2019.12.011
- Kim EJ, Kim N, Cho SG. MSCs in hematopoietic stem cell transplantation. Exp Mol Med. 2013;45(1):e2. doi: 10.1038/emm.2013.2
- Lee DW, Hong SW, et al. MSC cartilage regeneration after high tibial osteotomy. Orthop Traumatol Surg Res. 2025. doi: 10.1016/j.otsr.2025.104179
- Fraile M, Eiro N, et al. Aging and Mesenchymal Stem Cells. Biology. 2022;11(11):1678. doi: 10.3390/biology11111678


Interested in learning more? Discover our MSC eBook
Explore key insights into MSC cell culture, emphasizing the importance of media selection for cell therapy applications.
Related resources