Characterization of mesenchymal stem cells (MSCs) is vital for their successful application in regenerative medicine. These multipotent cells can differentiate into several cell types, making them a promising therapeutic option for various diseases. In our blog post, we discuss the importance of MSC characterization in ensuring standardized and reproducible cell isolation, self-renewal capacity, and differentiation.
Sources and characteristics of mesenchymal stem cells
Mesenchymal stem cells are a heterogeneous population of progenitor cells that can be derived from various tissue sources, including the bone marrow, adipose tissue, and umbilical cord. MSCs show stem cell characteristics, such as self-renewal and multipotency (ability to differentiate into multiple cell types) (Fig. 1).1,2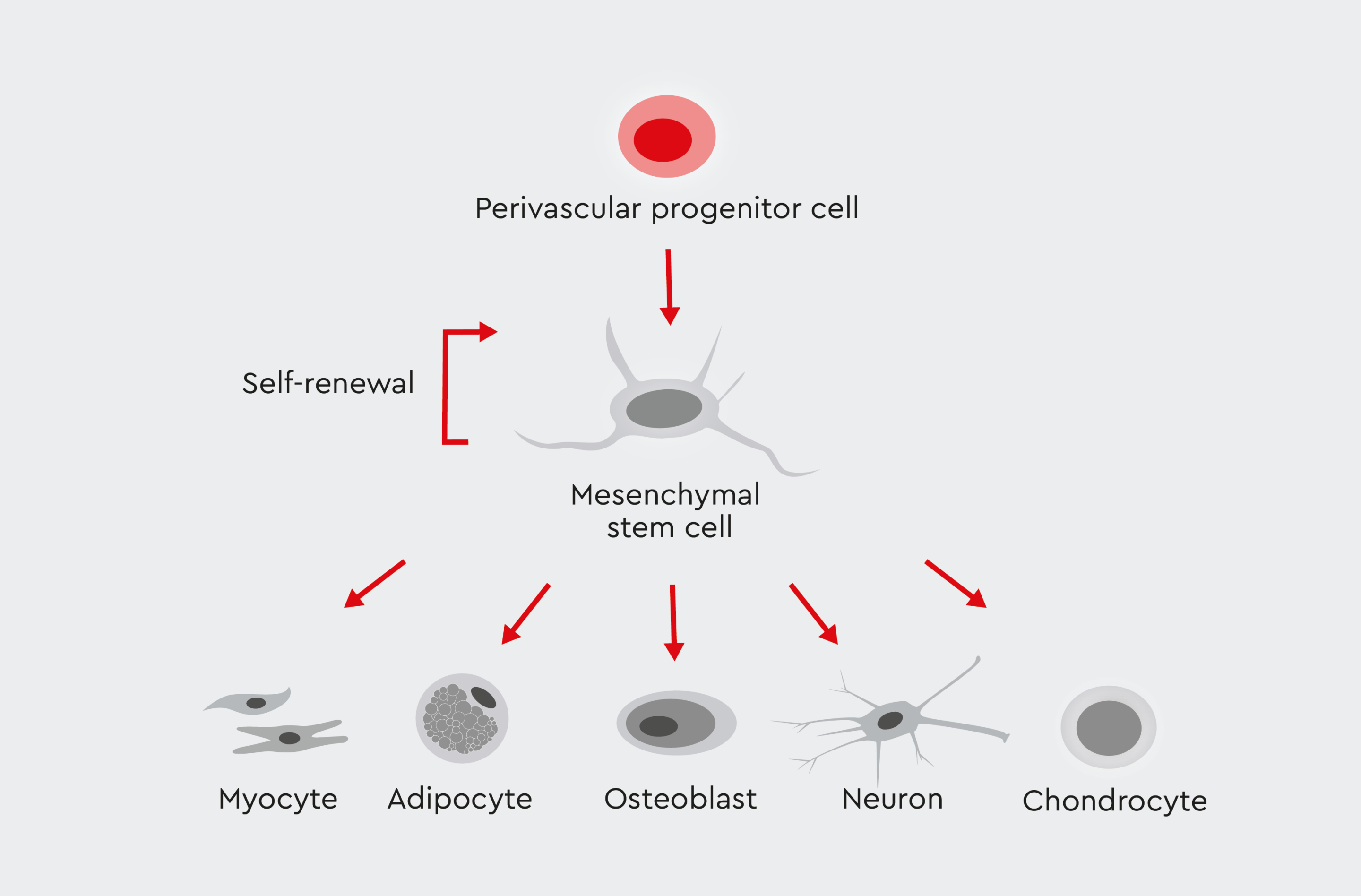
Figure 1: The differentiation lineage of mesenchymal stromal cells (MSCs).
MSCs have self-renewal capacity and are able to differentiate into multiple cell lineages.
Importance of mesenchymal stem cell characterization
Studies have shown that billions of dollars are spent each year in the United States alone on preclinical research that is not reproducible.5 Factors contributing to the lack of reproducibility in preclinical experiments include poor study design, use of different biological reagents, and inconsistent reference materials. The reproducibility of preclinical research can be improved by using validated materials and technologies and by adopting standardized practices for cell characterization. Proper characterization of MSCs ensures their identity, purity, and functional integrity. Accurate characterization is essential for:- Quality control: Robust characterization protocols are crucial for maintaining consistent and reproducible results in research and clinical applications.6
- Facilitating clinical translation: Accurate characterization is a prerequisite for the successful translation of MSC-based therapies from bench to bedside.7
- Comparability: Standardized characterization methods allow for the comparison of results across different studies and laboratories, promoting collaboration and advancing scientific knowledge.8
- Regulatory compliance: As MSC-based therapies progress toward clinical trials and eventual commercialization, rigorous characterization protocols are essential for meeting regulatory requirements and ensuring product quality and safety.9
MSC characterization guidelines
To provide a common set of standard criteria for MSC research, the International Society for Cell and Gene Therapy (ISCT)* has defined three minimum criteria to ensure accurate identification of human MSCs (Fig. 2)10:- Plastic adherence under standard culture conditions.
- Expression of specific surface markers (positive for CD105, CD73, and CD90; negative for CD45, CD34, CD14/CD11b, CD79α/CD19, and HLA-DR).
- Potential for differentiation into adipocytes, osteoblasts, and chondrocytes under standard in vitro conditions.
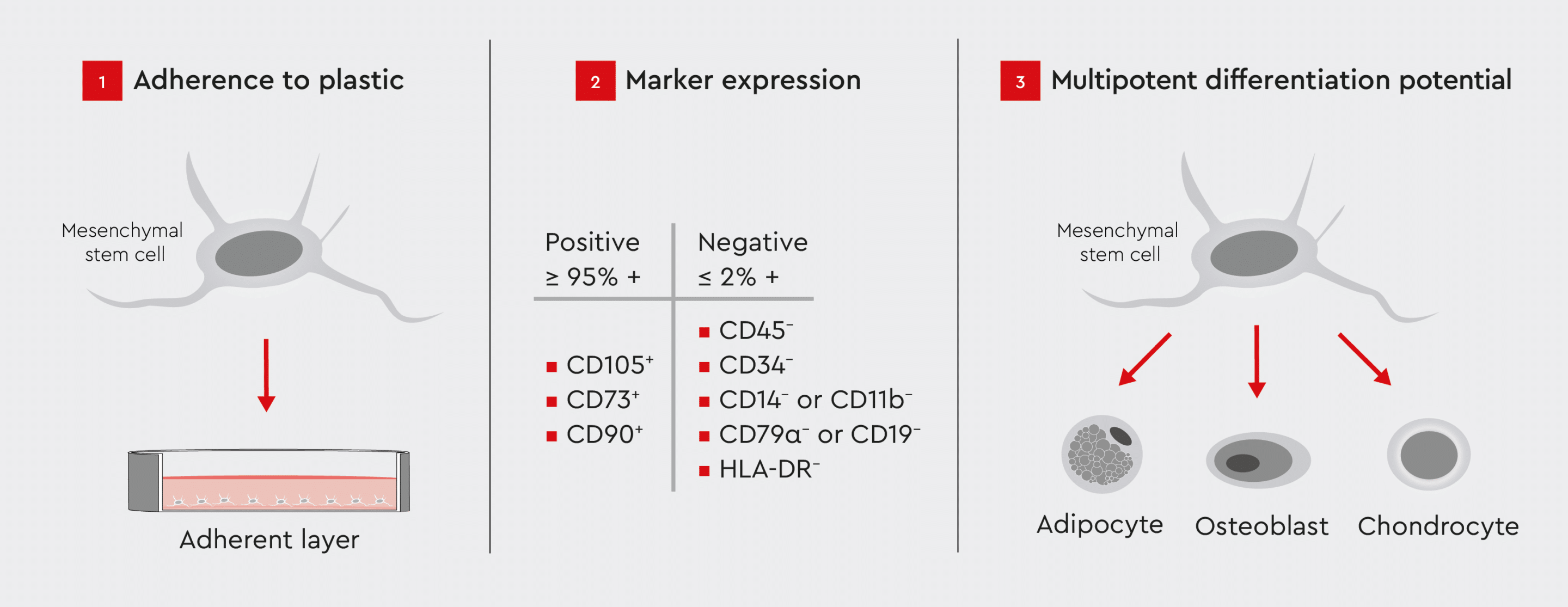
Figure 2: ISCT criteria for identifying MSCs for research purposes.
These criteria ensure the standardization and consistency of MSC identification in research.
* formerly known as International Society for Cellular Therapy
MSC characterization technologies
Various techniques are employed to validate the identity and function of MSCs. These include cell surface marker analysis, functional assays, and gene expression profiling.12 The selection of the characterization method depends on the features of the MSCs under question.MSC marker expression
MSCs are characterized by the expression of stem cell surface markers (e.g., CD90, CD105, CD73, CD44, and CD29) and lack of expression of hematopoietic and endothelial markers (CD45, CD31, CD34, and CD144).10 Flow cytometry is commonly used to analyze the expression of MSC-specific markers, enabling positive selection of cells expressing MSC markers and negative selection of cells expressing hematopoietic and endothelial markers.13 This process allows for the isolation of MSCs from a heterogeneous cell population. Characterization of MSC surface markers by flow cytometry following isolation from the bone marrow using our MSC Growth Medium showed a defined MSC population according to the recommended ISCT markers for determining MSC identity (Fig. 3).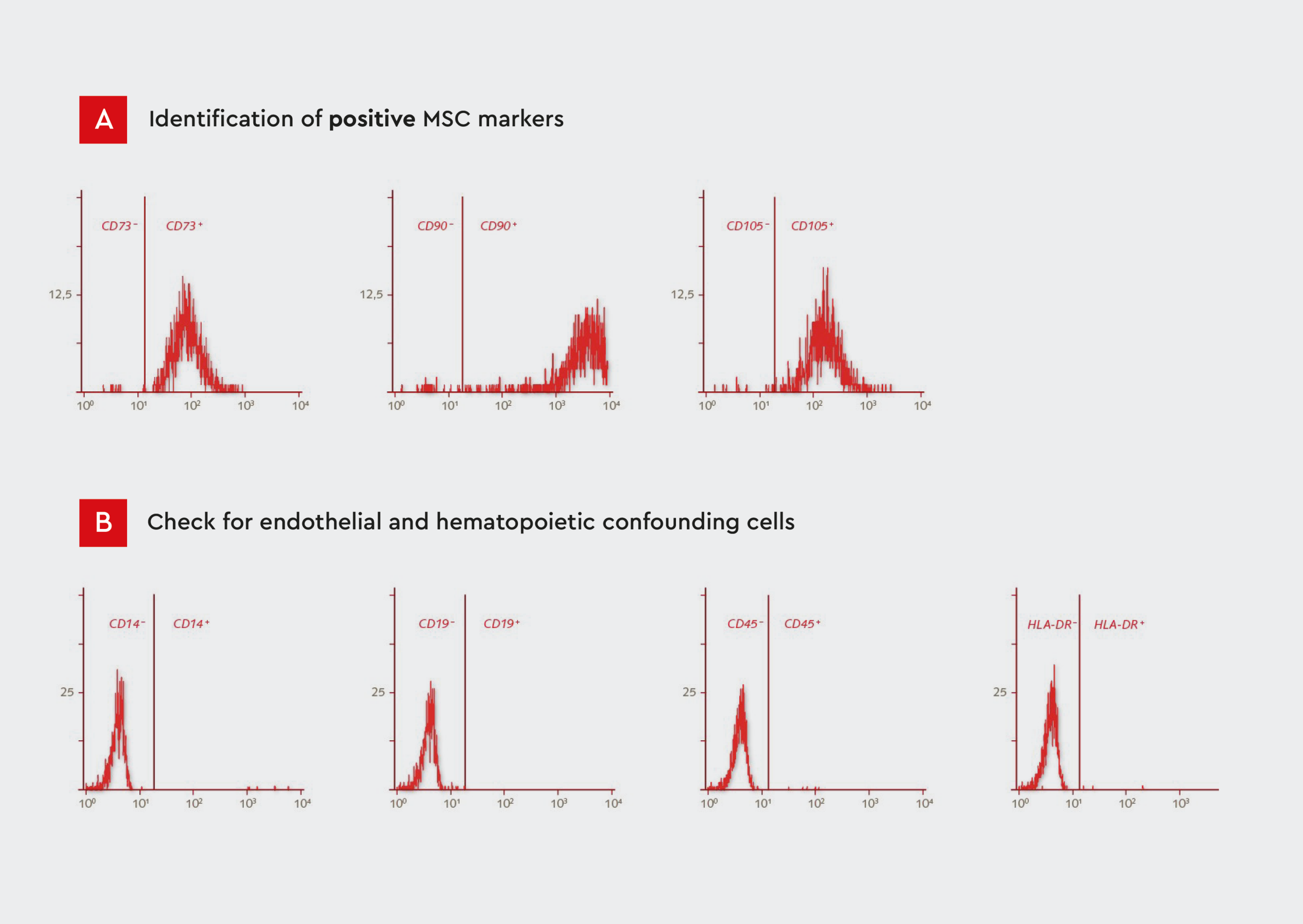
Figure 3: Flow cytometry analysis of our human primary mesenchymal cells isolated from bone marrow.
A) Histograms showing expression of CD73, CD90, and CD105. MSCs represent a defined population that is positive for these markers.
B) Histograms showing expression of CD14, CD19, CD45, and HLA-DR. MSCs are negative for these endothelial and hematopoietic markers. The expression profile conforms to the ISCT guidelines.
Self-renewal capacity
Like other stem cell populations, MSCs are characterized by self-renewal capacity. Colony-forming unit (CFU) assays are commonly used to evaluate the self-renewal and proliferative capacity of MSCs by quantifying their ability to form colonies from single cells seeded at low densities.14 Expansion of bone marrow-derived MSCs using our MSC Growth Medium XF resulted in a stable growth performance over several passages (Fig. 4). The MSCs used in this investigation demonstrated self-renewal capabilities, in accordance with the ISCT guidelines (see Fig. 2).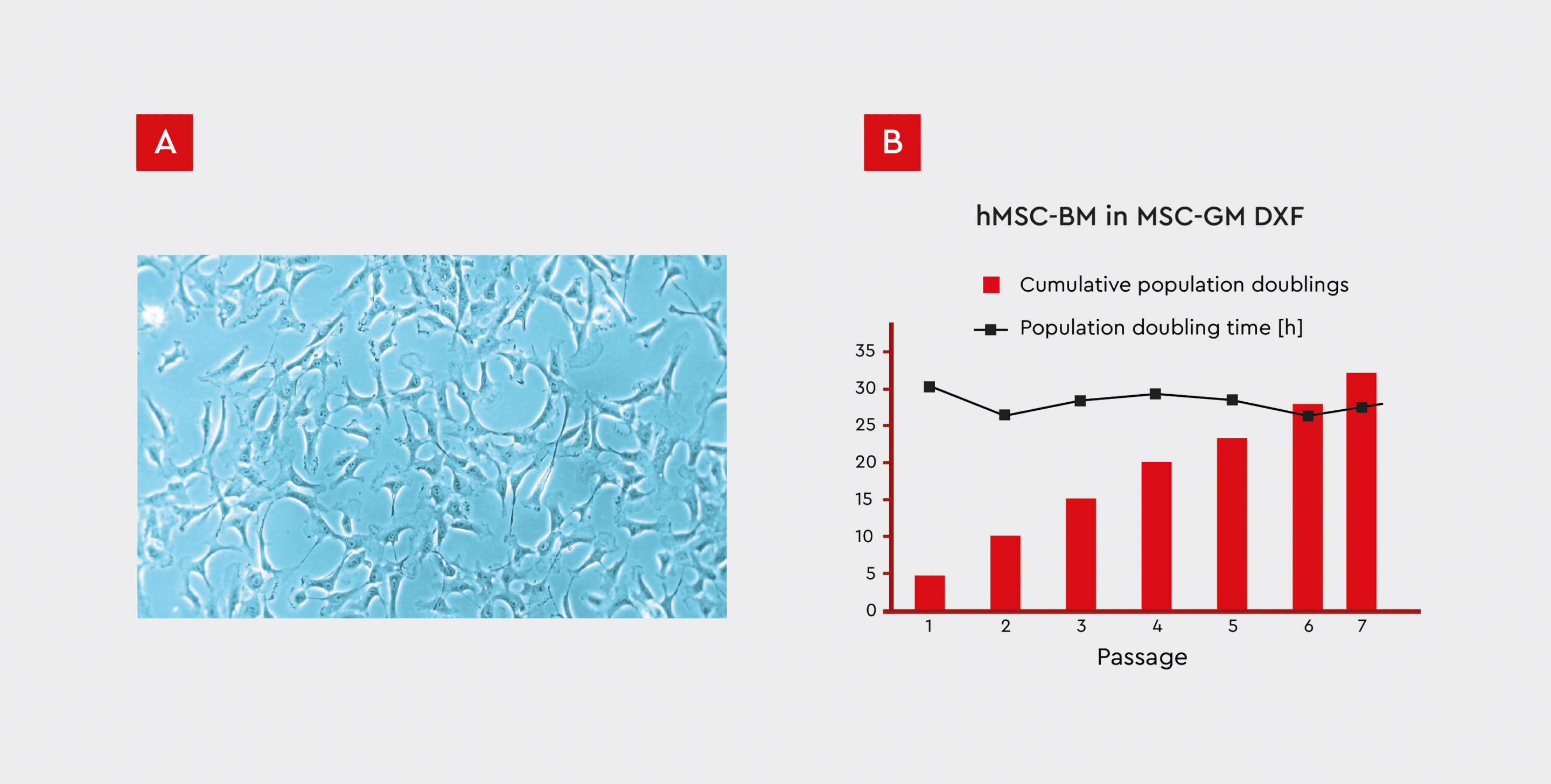
Figure 4: Growth performance of hMSCs isolated from bone marrow (hMSC-BM) cultured on fibronectin-coated plates.
A) MSCs were cultured in our Growth Medium DXF, which has a defined and xeno-free formula (MSC-GM DXF).
B) The cumulative numbers of population doublings and doubling times are shown over the course of 7 passages. A stable growth rate of less than 30 hours/doubling can be observed even after prolonged in vitro culture for 32 population doublings over the course of 7 passages.
Multipotency
MSCs are characterized by tri-lineage differentiation potential as they can differentiate into osteoblasts, chondrocytes, and adipocytes.10 Therefore, in vitro differentiation assays are commonly used to evaluate the ability of MSCs to differentiate into osteogenic (bone), chondrogenic (cartilage), and adipogenic (fat) lineages under defined culture conditions.15 Differentiation of expanded bone marrow MSCs into adipocytes, chondrocytes, and osteoblasts, in accordance with ISCT criteria, was assayed in passage-3 cells using our MSC differentiation media (Fig. 5). MSCs successfully differentiated into all three cell types, demonstrating their multipotency. Adipogenic differentiation resulted in cells with intracellular lipid vacuoles, typical of mature adipocytes (Fig. 5A). Formation of cartilage spheroids confirmed the chondrogenic differentiation of MSCs (Fig. 5B), and Alizarin Red S staining of extracellular calcium deposits in mineralized cells confirmed MSC differentiation into mature osteoblasts (Fig. 5C).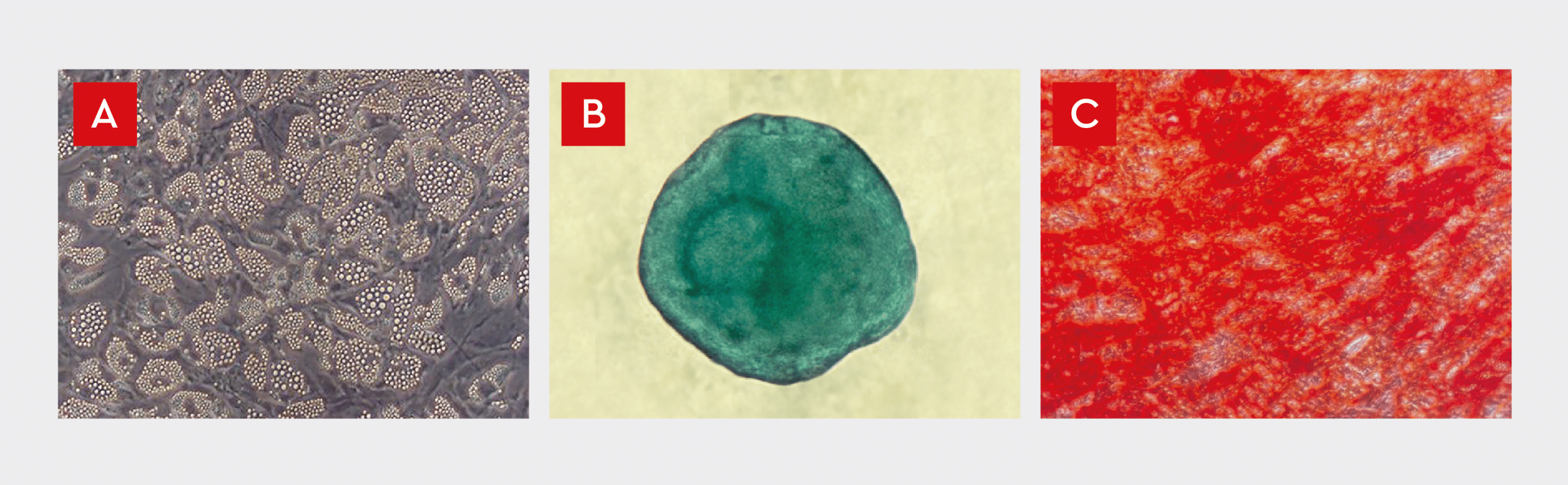
Figure 5: Differentiation of in vitro cultured our human MSCs into adipocytes (A), chondrocytes (B), and osteoblasts (C).
A) Lipid vesicle accumulation in adipocytes differentiated from human bone marrow-derived MSCs. The cells exhibit intracellular lipid vacuoles, typical of mature adipocytes.
B) Alcian Blue staining of MSC spheroids after in vitro differentiation into cartilage. Induced spheroids exhibit a blue color indicative of cartilage extracellular matrix.
C) Alizarin Red S staining of extracellular calcium deposits in mature osteoblasts differentiated from human bone marrow-derived MSC. The tri-lineage differentiation potential of our human MSCs is consistent with the ISCT guidelines (see Fig. 2).
Challenges in MSC characterization
Several challenges exist, including:- Heterogeneity within MSC populations: MSCs isolated from different tissue sources or even within the same tissue can exhibit significant heterogeneity in their surface marker expression, differentiation potential, and functional characteristics.4
- Variations in isolation and culture methods: The methods used for MSC isolation and culture can influence their properties and behavior, making it challenging to standardize protocols across different laboratories.6
- Influence of tissue source and donor age on MSC characteristics: The tissue source and age of the donor can impact the characteristics and functionality of the isolated MSCs, introducing variability in the cell populations.16
- Lack of standardized protocols for functional assays: While differentiation assays are commonly used, there is a lack of standardized protocols and quantitative measures for assessing the functional capabilities of MSCs, hampering cross-study comparisons.17
Our solutions for standardized MSC culture and characterization
The MSC isolation method and growth medium used for culturing play a crucial role in obtaining a well-defined MSC population that is in agreement with the ISCT guidelines. Standardization is essential for ensuring consistency, reproducibility, and comparability of MSC research and clinical applications. As cell culture experts, we provide high-quality MSCs and a comprehensive portfolio of specialized media for MSC culture, ensuring high-quality and reproducible results. These include:- Mesenchymal Stem Cell Growth Medium XF: A serum-free, xeno-free medium optimized for MSC expansion and characterization.
- Mesenchymal Stem Cell Growth Medium 2: A low-serum medium tailored for MSC culture and differentiation.
- PromoExQ MSC Growth Medium XF: A chemically defined, xeno-free medium designed for MSC expansion in a GMP-regulated environment.
Conclusions and future prospects
Mesenchymal stem cell characterization is a critical step in unlocking the full potential of these versatile cells in regenerative medicine. By adhering to standardized protocols and employing robust characterization techniques, you can ensure the identity, purity, and functional integrity of MSCs, facilitating reliable and reproducible results. As research in the MSC field continues to advance, the development of more comprehensive, inclusive, and harmonized characterization guidelines will be instrumental in accelerating clinical progress and in translating MSC-based therapies into real-world applications. Advancements in technology will likely lead to more robust and efficient methods for MSC characterization, such as single-cell analysis for deeper insights into cellular heterogeneity. Moreover, a deeper understanding of MSC biology will pave the way for strategies to improve their therapeutic potential, such as preconditioning them to enhance their homing and engraftment capabilities.18References
Expand
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287-2299. doi:10.1634/stemcells.2007-1122
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44(4):215-230.
- Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113(9):2806-2812.
- Costa LA, Eiro N, Fraile M, et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. 2021;78:447-467.
- Freedman LP, Cockburn IM, Simcoe TS. The economics of reproducibility in preclinical research. PLoS Biol. 2015;13(6):e1002165. doi:10.1371/journal.pbio.1002165
- Wright A, Arthaud-Day ML, Weiss ML. Therapeutic use of mesenchymal stromal cells: the need for inclusive characterization guidelines to accommodate all tissue sources and species. Front cell Dev Biol. 2021;9:632717.
- Lui PPY, Leung YT. Practical considerations for translating mesenchymal stromal cell-derived extracellular vesicles from bench to bed. Pharmaceutics. 2022;14(8):1684.
- Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173-2185.
- de Almeida Fuzeta M, de Matos Branco AD, Fernandes-Platzgummer A, da Silva CL, Cabral JMS. Addressing the manufacturing challenges of cell-based therapies. Curr Appl Pharm Biotechnol. 2020:225-278.
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. doi:10.1080/14653240600855905
- Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So. Cytotherapy. 2013;15(6):641-648. doi:10.1016/j.jcyt.2013.02.006
- Ranera B, Lyahyai J, Romero A, et al. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 2011;144(1-2):147-154.
- Kouroupis D, Churchman SM, English A, et al. Assessment of umbilical cord tissue as a source of mesenchymal stem cell/endothelial cell mixtures for bone regeneration. Regen Med. 2013;8(5):569-581.
- Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4(8):e6498.
- Gimble JM, Guilak F, Nuttall ME, Sathishkumar S, Vidal M, Bunnell BA. In vitro differentiation potential of mesenchymal stem cells. Transfus Med Hemotherapy. 2008;35(3):228-238.
- Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom Part A. 2018;93(1):19-31.
- Galipeau J, Krampera M, Barrett J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151-159.
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2). doi:10.1042/BSR20150025
Contact our experts
Reach out to our experts to learn more about our MSCs and specialized cell culture media.
Contact us


MSC reproducibility: Towards the standardization of Mesenchymal Stem Cells
For science to move forwards, the research we do must be reproducible. One of the ways we’re helping your work be as consistent as possible is through our characterized MSCs….
