Mesenchymal stem cells: why optimizing manufacturing processes is key for a successful application
Discover the reasons why an optimized manufacturing process is essential for successful application of mesenchymal stem cells.
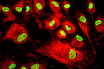
Mesenchymal stem cells (MSCs) represent a promising avenue for therapeutic development, with their potential to treat a wide range of diseases. Despite the considerable advantages MSCs show, the lack of efficient manufacturing processes has made clinical translation of MSCs more challenging.
In this white paper, you will learn more about:
- stumbling blocks to large-scale MSC production
- various media, methods, and processes
- why consistent protocols are essential for MSC therapy
- ensuring quality, safety, and efficacy
Download the white paper
Fill in the form to download the PDF.
Related resources
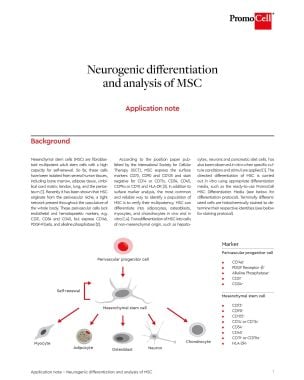
Neurogenic differentiation and analysis of mesenchymal stem cells (MSC)
Specific culture conditions allow for transdifferentiation of mesenchymal stem cells (MSC) into cells of non-mesenchymal origin such as neurons. To do so, specific culture conditions and stimuli are needed.
Application Note

Chondrogenic differentiation and analysis of mesenchymal stem cells (MSC)
Managed in vitro differentiation of mesenchymal stem cells (MSC) into chondrocytes. Multipotent adult stem cells are important for various tissues of the musculoskeletal system.
Application Note

Adipogenic differentiation and analysis of mesenchymal stem cells (MSC)
Turning mesenchymal stem cells (MSC) into adipocytes is the aim of this application note. Research using MSC is a field of high interest and could ultimately lead to new therapies for treating a variety of lifestyle diseases.
Application Note

Xeno-free MSC culture: high yield, better viability and morphology
Are you trying to mimic in vivo conditions in your MSC cultures as closely as possible, but need to avoid serum? Then find out how the combination of PromoCell DXF medium and a unique coating called myMATRIX help you make your conditions highly physiological while maintaining reproducibility.
Application Note

MSC cultivation in single-use stirred tank bioreactors
MSCs have gained significant attention because of their therapeutic potential for many difficult-to-treat diseases. In this app note, we provide a protocol for (GMP) compliant large-scale production of MSCs in a single-use bioreactor using our MSC Growth Medium XF.
Application Note

Mesenchymal stem cells: a cell culture handbook
Discover key insights into MSC cell culture, emphasizing the importance of media selection for cell therapy applications.
Application Note

Osteogenic differentiation and analysis of mesenchymal stem cells (MSC)
Differentiate mesenchymal stem cells (MSC) into osteoblasts. Read our application note and find out more. The multipotency of these cells is one essential characteristic of MSC, according to the International Society for Cellular Therapy.
Application Note
Recommended products
Mesenchymal Stem Cell Adipogenic Differentiation Medium 2
Cell culture medium for differentiation of human mesenchymal cells into adipogenic lineages.
284.00 CHF
Mesenchymal Stem Cell Chondrogenic Differentiation Medium
Serum-free cell culture medium for differentiation of mesenchymal cells into chondrogenic lineages.
From
661.00 CHF
Mesenchymal Stem Cell Growth Medium 2
Low-serum cell growth medium for mesenchymal stems cells.
357.00 CHF
Mesenchymal Stem Cell Neurogenic Differentiation Medium
Serum-free cell culture medium for differentiation of mesenchymal cells into neurogenic lineages.
560.00 CHF
Mesenchymal Stem Cell Osteogenic Differentiation Medium
Cell culture medium for differentiation of mesenchymal cells into osteogenic lineages.
284.00 CHF

Human Mesenchymal Stem Cells (hMSC)
Primary Human Mesenchymal Stem Cells. Available from adipose tissue, bone marrow and umbilical cord matrix.
From
1’709.00 CHF