Oxidative stress results from an imbalance of oxidants and antioxidants and promotes skin aging, inflammation, and disease. Cell assays and primary cells allow researchers to study oxidative processes and their associations with skin aging.
Aging is an inevitable process experienced by most organisms and results in progressive functional decline. One of the most apparent signs of aging in humans is changes in the appearance of the skin, which becomes thinner, less elastic, wrinkled, and less able to perform its barrier function.1, 2 Skin aging is a multifaceted process, and researchers are still attempting to fully understand the complex mechanisms that promote skin changes during aging. Skin aging is regulated by both intrinsic and extrinsic factors:- Intrinsic factors are largely genetically determined and promote skin atrophy, increased vascular prominence, reduced elasticity, and fine wrinkles.
- Extrinsic factors include exposure to ultraviolet (UV) light and pollution, promoting deep wrinkles, rough texture, telangiectasia, and pigmentation irregularities. Different skin types respond differently to extrinsic factors, with type I (white) and type II (fair) skin experiencing increased extrinsic aging in response to the same stimuli.1
The causes of oxidative stress
Although oxidative stress accelerates aging, oxidative processes are also essential for energy production. Oxidative stress is commonly defined as an imbalance between pro- and antioxidants.3 Oxidants include free radicals, reactive oxygen species (ROS), and peroxides, while antioxidants include glutathione (GSH) and superoxide dismutase (SOD). The most significant extrinsic factor accelerating skin aging is exposure to UV light, leading to ROS production and oxidative damage.4 Lipids, proteins, carbohydrates, and DNA are susceptible to oxidative damage, which can modulate gene transcription, cell proliferation, and differentiation. Oxidative damage is involved in several diseases, including diabetes and obesity, cancer, and neurological disorders.5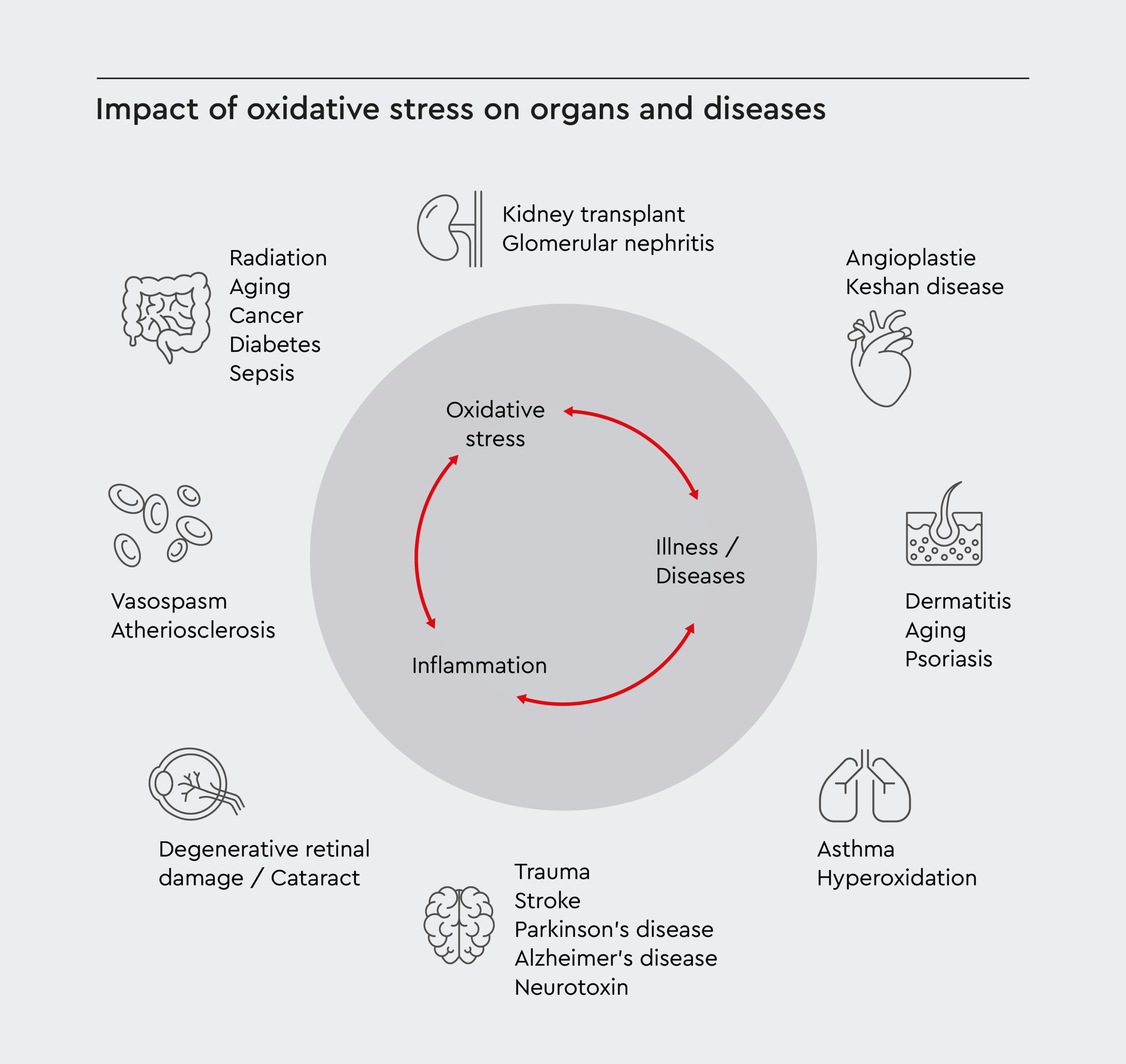
Figure 1: Impact of oxidative stress on organs and diseases.
The circular scheme shows how oxidative stress and inflammation interact and reinforce each other. These processes can lead to various medical conditions, including neurological disorders, cardiovascular issues, kidney problems, aging, and cancer.
How does oxidative stress affect skin aging?
Research suggests that aging is caused by cumulative oxidative stress due to free radicals generated via metabolism and as a result of exposure to UV light.4 Free radicals, both ROS and reactive nitrogen species (RNS), can cause damage to biomolecules, influencing gene expression, cell metabolism, and physiological functions.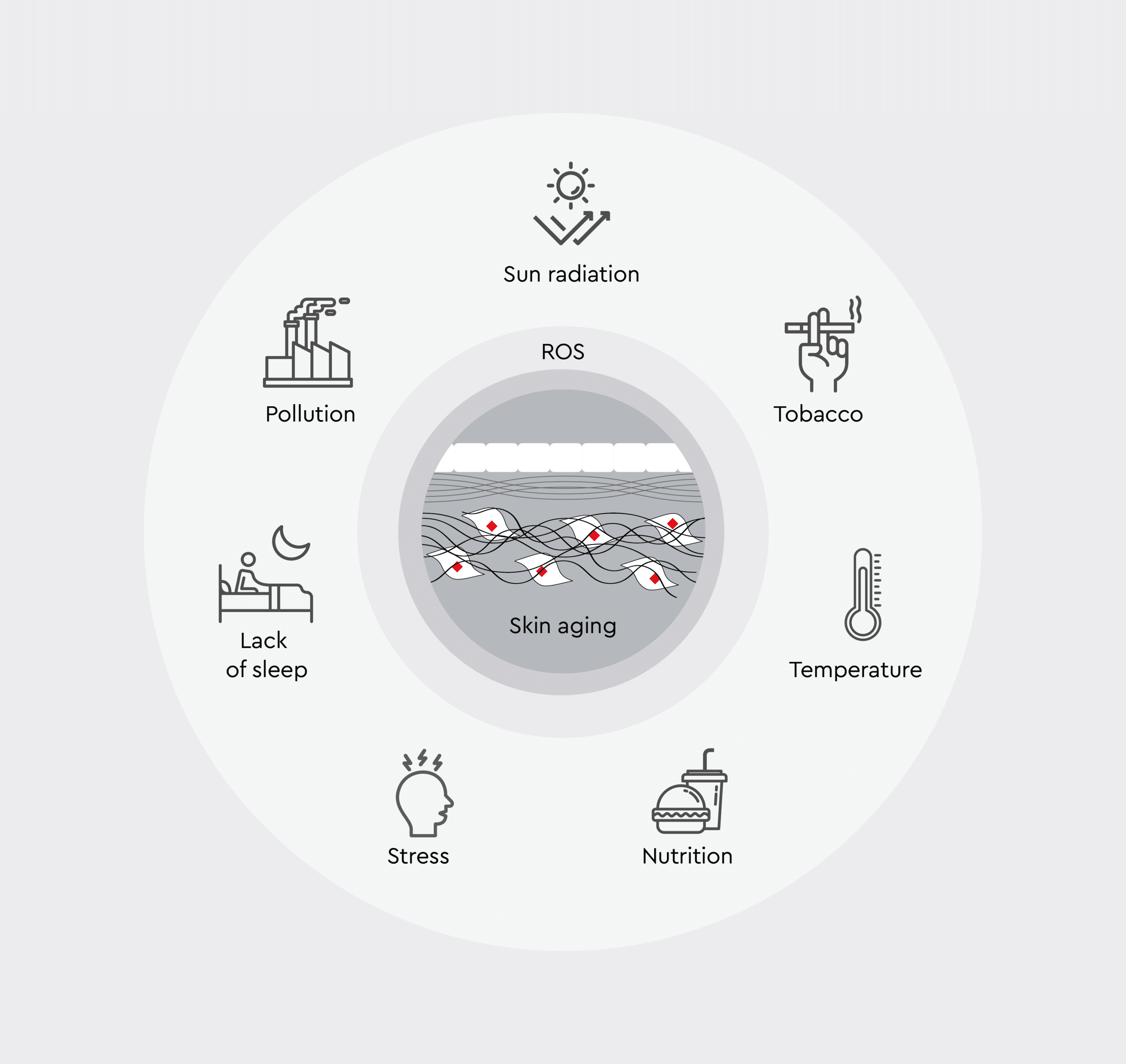
Figure 2: Formation and impact of ROS.
Overview of factors contributing to ROS production in skin, highlighting the interplay between environmental, physiological, and lifestyle influences that accelerate skin aging through oxidative stress.
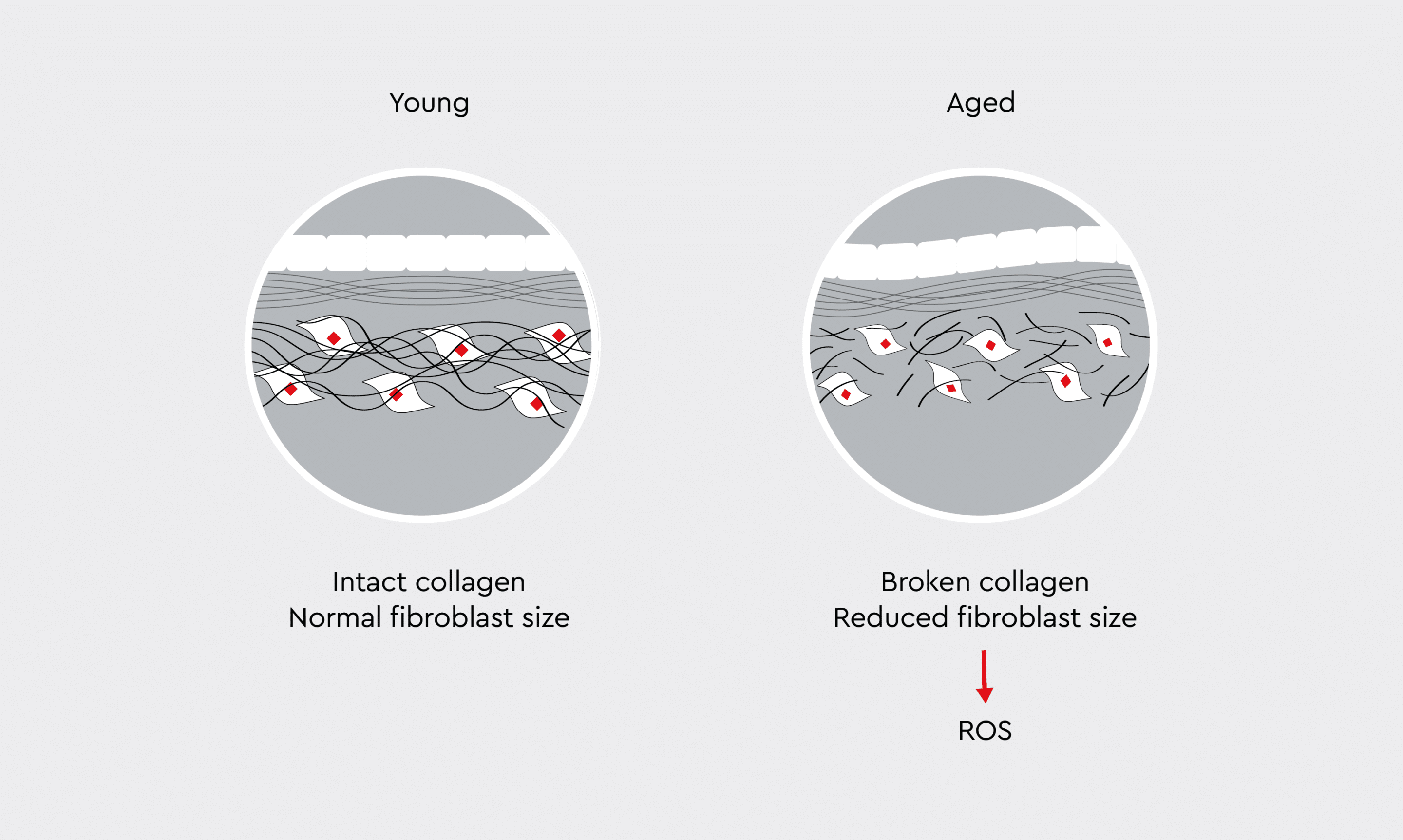
Figure 3: Collagen breakdown caused by oxidative stress.
Young skin contains an intact, well-organized collagen network, normal-sized fibroblasts, and dense extracellular matrix. In contrast, aged skin is characterized by fragmented collagen fibers, reduced fibroblast size, and a more disorganized matrix structure.
Role of telomeres in oxidative stress-induced skin aging
ROS can accelerate telomere shortening, particularly because telomeres’ TTAGGG repeats are rich in guanine bases, which are vulnerable to oxidative modification.9 Skin cell types respond differently to oxidative stress. Keratinocytes treated with low levels of peroxide show delayed telomere shortening and extended lifespan through antioxidant modulation. In contrast, peroxide treatment in fibroblasts increases telomere shortening, thereby reducing lifespan. Additionally, keratinocytes are more resistant to DNA damage and tend to undergo apoptosis. On the other hand, damaged fibroblasts become senescent in the dermal tissue and can affect epidermal growth through processes such as paracrine signaling.9 This suggests that oxidative stress contributes to skin aging through both direct telomere damage and cellular senescence pathways.Commonly used assays to study oxidative stress
Several assays are available to study the effects of oxidative stress on DNA damage, the redox state, and cell proliferation and survival. In vitro assays are also commonly used to study the cellular redox balance, including oxidant and antioxidant levels, and to screen drug candidates for the development of therapies targeting oxidative stress and skin aging. To enable these applications, assays must be highly sensitive, reproducible, easy to use, and suitable for high-throughput screening. Oxidative stress assays can be used to investigate the following:- Glutathione metabolism
- Antioxidant/anti-aging
- ROS production
- Nitrite and nitric oxide metabolism
- Oxidase/peroxidase activity
- DNA damage
Commonly used primary cells and cell models to study the role of oxidative stress and skin aging
Primary cells and cell models are commonly used to study oxidative stress, aging, and the complex crosstalk between different cell types in the skin. The latter requires the use of three-dimensional approaches, such as organotypic skin models, which allow the coculture of different skin cell types in a three-dimensional matrix to create a life-like model.10 Three-dimensional skin models can be adapted to better understand specific skin types. For example, it is possible to develop 3D organotypic cultures that mimic the aged skin.10 In vitro skin aging models can also be used to determine gene expression levels and cell growth rate in skin cells, allowing for molecular studies of skin cell changes during aging and drug screening.7Looking forward
The aging of the global population has increased the demand for research into factors affecting skin aging and prevention of aging. As research progresses, there will be an increased focus on realistic models that mimic the complex skin structure and allow the screening of pharmacologic compounds. Ideal skin models are required to enable 3D cell culture and high-throughput screening. Developing realistic and reproducible skin aging models could help not only the cosmetics industry, but also those studying skin diseases and testing new therapies. Aging of the skin affects multiple physiological processes and diseases throughout the body.References
Expand
-
- Naidoo K, Birch-Machin MA. Oxidative stress and ageing: the influence of environmental pollution, sunlight and diet on skin. Cosmetics 2017;4:4
- Oliveira BF, Nogueira-Machado JA, Chaves MM. The role of oxidative stress in the aging process. The Scientific World Journal 2010;10:1121-1128
- Tan BL, NOrhaizan ME, Liew WPP, Rahman HS. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Frontiers in Pharmacology 2018;9:1162
- Stojiljkovic D, Pavlovic D, Arsic I. Oxidative stress, skin aging and antioxidant therapy. Scientific Journal of the Faculty of Medicine in Nis 2014;31(4):207-217
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol 2017;292:18-36
- Jomova, K., Alomar, S.Y., Alwasel, S.H. et al. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol 2024; 98:1323–1367
- Carvalhaes Lago J, Puzzi MB. The effect of aging in primary human dermal fibroblasts. PLoS One 2019;14(7):e0219165
- Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, Zhou T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int J Mol Sci 2019; 4472
- Buckingham EM, Klingelhutz AJ. The role of telomeres in the ageing of human skin. Exp Dermatol 2011;20(4):297-302
- Weinmullner R, Zbiral B, Becirovic A, et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. Aging and Mechanisms of Disease 2020;6:4
Contact us for more information
Advance your dermatological research with our comprehensive portfolio of matched donor cell types. Contact our specialists today.
Contact us