In this interview with RegMedNet, our expert, Dr. Elfie Rödel discusses the relevance of the air-liquid interface (ALI) culture system in regenerative medicine and the outlook for ALI-culture applications in the future. Dr. Rödel also explores the cell lines that can be utilized for ALI techniques and what factors should be considered when choosing a model cell line.
Why is the Air-liquid interface (ALI) technique increasingly relevant in regenerative medicine today?
The rise in respiratory diseases like chronic obstructive pulmonary disease (COPD) and lung cancer, driven by aging populations and pollution, underscores the need for advanced therapeutic approaches. Lung tissue has limited regenerative capacity, with airway epithelial cells renewing at less than 1% per day, making treatments for conditions like interstitial pneumonia rare.
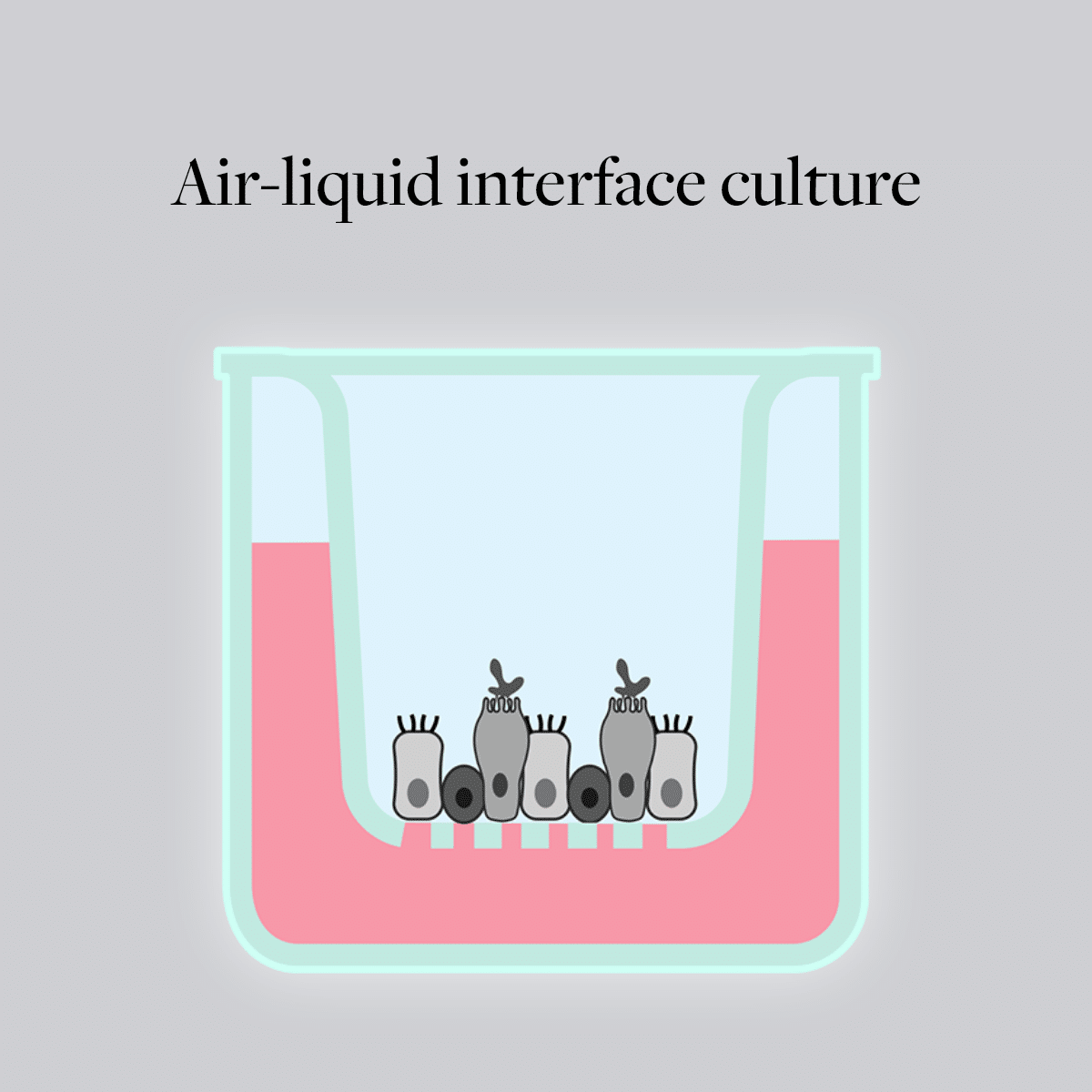
ALI cultures using human airway epithelial cells offer an accurate model for studying lung diseases, compared with traditional animal models, which have a number of limitations due to significant physiological differences between species and humans. This technique involves culturing cells at the interface of air and a nutrient medium, which closely mimics the natural environment of epithelial tissues like those in the respiratory tract and the skin.
ALI cultures have gained traction over the past 20 years, while these methods are advocated by the European Union and the American FDA to reduce animal experiments in science. In regenerative medicine, efforts are underway to treat lung diseases with personalized approaches, including the potential use of engineered tissue fragments from patient cells. Early studies using autologous lung tissue are promising. This highlights the potential of ALI cultures in translating research into practical regenerative medicine treatments.
How does the ALI technique compare to traditional submerged cell culture methods in terms of replicating in vivo conditions?
Due to the exposure to ambient air, cells in ALI cultures have greater access to atmospheric gases than they do in submerged cultures. This exposure causes changes in oxidative metabolism and promotes cellular differentiation. Depending on the cell type used, cilia-bearing cells and mucus-producing cells can occur in ALI culture. This is not common under submerged culture conditions. Furthermore, the ambient air can affect the morphology and polarization of airway epithelial cells. For example, primary human nasal epithelial cells (HNEpCs) are flattened in traditional submerged cultures. In living organisms, the polarization of epithelial cells is a key characteristic. Under ALI conditions, HNEpCs can develop a comparable number of cilia with comparable ciliary beating frequency as observed in ex vivo biopsy samples. The adapted structure of airway epithelial cells, including a functional physical barrier with cilia beating and mucus production, makes ALI culture preferable to traditional 2D culture. In contrast to submerged cultures, test substances can be added directly to the cells in ALI cultures. This simulates the application of pulmonary therapeutic approaches like inhalation, nebulizers or aerosols. ALI cultures enable efficient high-throughput screenings for inhalation studies and pulmonary toxicology. By using co- or tri-cultures of different cell types, researchers can better study the cellular interactions within the airway wall. It is also possible to separate different cell types locally from each other by seeding cells on the basolateral or apical side of the porous membrane, which is not possible in traditional 2D culture. ALI cultures provide a higher level of structural complexity and diversity of cell populations that cannot be achieved in submerged culture.What types of cell lines are commonly used in ALI and what are the critical factors to consider when selecting a model cell line for ALI cultures?
Various types of model cells are commonly utilized in ALI cultures for questions in regenerative medicine. These include primary somatic cells derived from the respiratory tract, isolated human stem cells (e.g. from bone marrow) or immortalized airway-derived cell lines. When selecting a cell type as model for ALI cultures, several critical factors should be considered:- Origin and Functionality: Choose a cell type that closely mimics the tissue of interest in terms of origin and function. For example, HBEpCs are suitable for studying diseases affecting the bronchi, while the cell line A549 are used to model alveolar functions.
- Differentiation Capacity: Assess the cell types’s ability to differentiate into mature epithelial cells under ALI conditions. This is crucial for creating physiologically relevant models.
- Genetic Stability: Consider the genetic stability of the cell line over passages, as genetic abnormalities can affect experimental reproducibility and relevance to human biology.
- Barrier Integrity: Evaluate the transepithelial electrical resistance (TEER) of the cell type when grown on porous membranes. Higher TEER values indicate better barrier integrity, which is important for studying drug permeability and mucociliary clearance. HNEpCs with TEER values higher than 1,000 Ω*cm2 are best suited for this application.
- Response to Stimuli: Understand how the model cell responds to physiological stimuli or disease-relevant factors (e.g., cytokines, pathogens) to ensure it reflects the intended research objectives.
Are there any challenges associated with optimizing ALI?
Selecting the right airway epithelial cell plays a major role in the sensitivity of the ALI culture. Another important consideration is the selection of suitable cell culture plastics, particularly the porous membrane material. There are various suppliers of semipermeable membranes in multiwell plate format available. Common materials include PET, available in clear plastic, which can be used under microscopes, and translucent plastic, which are not suitable for microscopy. Collagen pre-coated polycarbonate or polyester membranes are also available. Pore size is critical, especially for studies involving drug transport and cell migration. Pore density can vary by manufacturer, affecting nutrient availability from the medium. The physical properties of the porous membrane — topography, stiffness and free ligand availability — can influence cell adhesion and morphology, impacting experimental outcomes. The culture medium must support passive diffusion and ion channel activity, reflecting the cells’ microenvironment, with factors like pH and buffer systems influencing the system. Optimizing the microclimate in the ALI chamber involves controlling ambient temperature, humidity, electrostatic forces and membrane surface hydrophobicity. Additionally, the culture medium’s characteristics, such as osmolality and viscosity and the aqueous lining of mucus and surfactant lipid layer can affect the diffusion and reaction kinetics of test agents during exposure tests. Traditional ALI culture is static, but since mucus circulates in the lungs via mucociliary clearance, dynamic cultures better reflect physiological conditions. Therefore, efforts are underway to integrate dynamic elements that replicate biomechanics, shear stress or cyclic stretch. Dynamic cultures can alter the cellular mechanotransduction, which in turn can lead to biological effects. Solutions include lung-on-a-chip models that imitate the mechanical forces during regular breathing movements, optimized breathing lung bioreactors and dynamic in vitro stretch models for the alveolar interface.What do you envision for the future of ALI technology in regenerative medicine?
The future of ALI technology in regenerative medicine is promising. Complex triculture systems, incorporating airway epithelial cells and fibroblasts, smooth muscle cells or endothelial cells, will create detailed airway wall models. Adding immune cells will help model the pulmonary innate immune system, potentially leading to new treatments for COPD, asthma, cystic fibrosis and viral infections.
ALI will enable accurate disease models, integrate with organoids and lab-on-a-chip systems and advance tissue engineering for bioengineered tissues. For instance, scientists have used ALI cultures with human iPSC-derived alveolar type 2 cells to model COVID-19 and test SARS-CoV-2 infection. Stem cell applications will expand, leading to personalized regenerative therapies using autologous patient samples.
Enhanced, reliable in vitro testing platforms will reduce reliance on animal models, supporting faster and more predictive toxicity assessments. Clinical translation and commercialization will make regenerative treatments more accessible, aligning with regulatory goals to reduce in vivo testing. Consequently, regenerative medicine will revolutionize diagnostic and therapeutic approaches across various diseases.
Find the entire In Focus on air-liquid interface (ALI) culture on RegMedNet.