The functions of fibroblasts in skin extend beyond basic biological functions, making them promising targets for treating various skin conditions. These versatile cells orchestrate wound healing, tissue regeneration, and skin homeostasis. Despite the significant progress in fibroblast research, there are still challenges to overcome, such as the limited availability of research-specific fibroblasts and inconsistencies in culture media. Read on to learn how innovative solutions like the use of defined and animal component-free (D-ACF) media are addressing these issues.
The role of fibroblasts in skin biology
Dermal fibroblasts are specialized cells that maintain the structure and function of the skin (Figure 1). They originate from mesenchymal cells and are primarily located in the dermis, the layer of skin beneath the epidermis.1 These cells have tissue-specific properties that differ from those of fibroblasts found in other organs.2 Their unique properties make them invaluable for skin biology-related research. Skin fibroblasts produce several extracellular matrix (ECM) components, including type I and III collagen, elastin, proteoglycans, and glycosaminoglycans. By doing so, they help maintain skin architecture and elasticity.3 In addition to producing ECM components, they also secret various regulatory molecules that help maintain skin function. These molecules include growth factors, cytokines, matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs).4 By secreting ECM components, growth factors, and cytokines, they help maintain skin barrier function, support epidermal regeneration, and modulate immune responses in the skin.4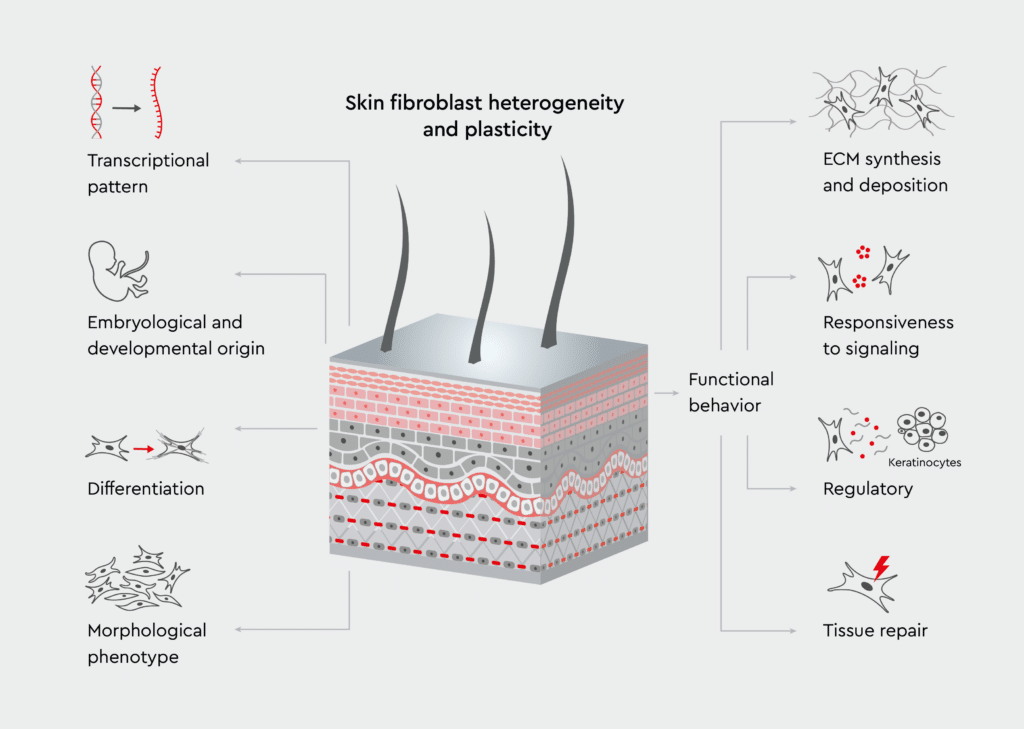
Figure 1: Role of fibroblasts in maintaining the architecture of the skin.
The scheme illustrates fibroblasts within the skin layers, highlighting their interaction with the surrounding cells and ECM components. Adopted from Knoedler et al., 2023.5
Fibroblasts and wound healing
Wound healing is a fascinating process, involving tissue regeneration, ECM production, and several cellular interactions. In this section, we explore the phases of healing and the critical role fibroblasts in skin play at each stage.The wound healing cascade
How do fibroblast cells help in wound healing? When skin injury occurs, skin fibroblasts are activated and initiate a wound repair cascade consisting of three phases (Figure 2):- Immediate response phase (hemostasis/inflammation): Upon injury, fibroblasts are activated by cytokines and growth factors released during the inflammatory response. In turn, activated fibroblasts upregulate the expression of cell surface receptors and adhesion molecules.10,11
- Migration and proliferation: Guided by chemotactic signals, activated fibroblasts migrate to the injury site and proliferate to populate the wound area. Along with other cells, activated fibroblasts release various growth factors that promote angiogenesis, re-epithelialization, and recruitment and activation of other cell types involved in wound healing.11,12
- Matrix remodeling: Fibroblasts in skin differentiate into myofibroblasts, which exhibit increased production of ECM components and MMPs and enhanced deposition of collagen.12 This process leads to the formation of provisional matrix, a fibrin-containing molecular scaffold that supports blood clotting and wound healing.13
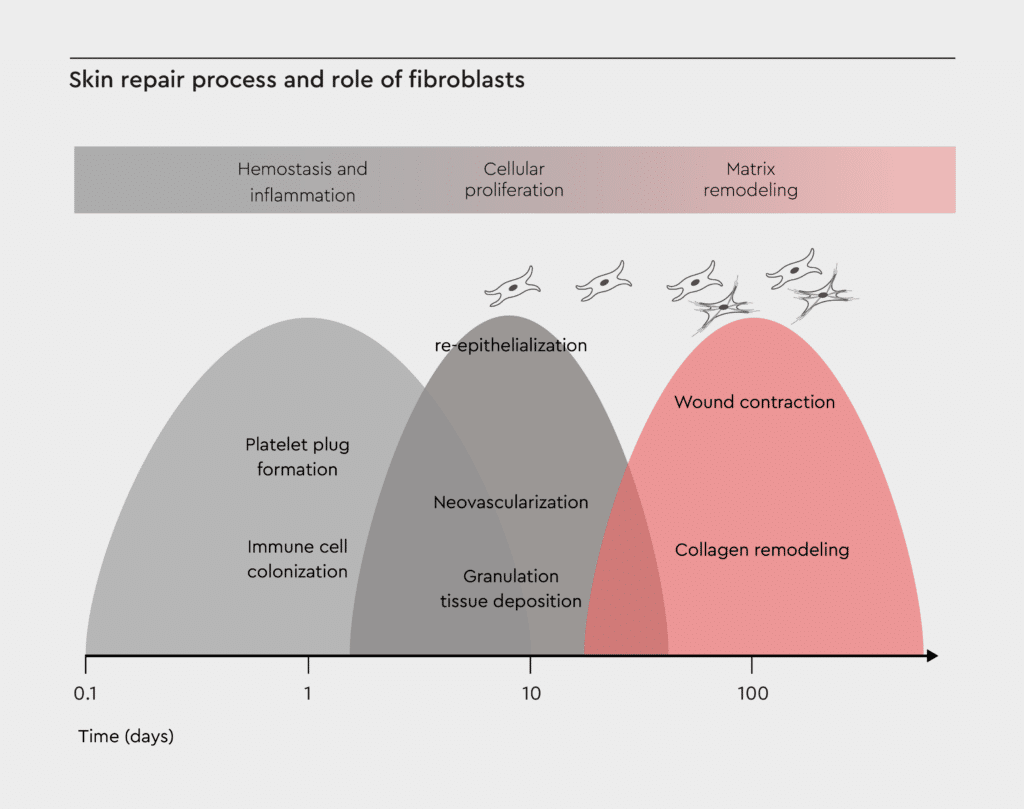
Figure 2: Skin repair process and role of fibroblasts.
This diagram shows the three overlapping phases of wound healing: hemostasis/inflammation, proliferation, and matrix remodeling. Skin fibroblasts contribute to ECM production, growth factor secretion, and tissue remodeling. Adopted from Castaño et al., 2018.12
Growth factor signaling and cellular crosstalk in wound repair
Fibroblast cells in skin secrete and respond to numerous growth factors during wound healing:- Platelet-derived growth factor (PDGF)
- Transforming growth factor-β (TGF-β)
- Vascular endothelial growth factor (VEGF)
- Basic fibroblast growth factor (bFGF)
Regeneration versus scarring: A fine balance
Wound healing and tissue regeneration involve balanced ECM production, controlled inflammation, proper vascularization, and organized matrix alignment.14 Deregulation of one or more of these processes can lead to pathological scarring. Factors contributing to excessive scarring include prolonged inflammation, excessive myofibroblast activation, imbalanced ECM deposition, and altered wound environment (e.g., mechanical forces, pH, oxygen levels, and cytokine and growth factor milieu).15,16 Although fibroblasts are essential for wound healing, they also play a role in scar formation by coordinating with other cells during the resolution phase to restore skin integrity after injury.1 Understanding the mechanisms regulating the balance between regeneration and scarring is crucial for developing therapies that promote optimal healing with minimal scarring.From lab to clinic: Fibroblast-based therapies
The unique properties of skin fibroblasts make them attractive candidates for cell-based therapies in various therapeutic areas.Examples of approved fibroblast-based therapies for skin conditions
Currently, three FDA-approved dermal fibroblast-based therapies are available:- LAVIV (azficel-T): FDA-approved autologous fibroblast therapy, used for the improvement of the appearance of moderate to severe nasolabial fold wrinkles. It involves culturing a patient’s own fibroblasts and injecting them back into the skin to promote collagen production.18
- GINTUIT: This product comprises allogeneic cultured keratinocytes and fibroblasts in bovine collagen and is used to generate new and healthy gum tissue in patients with gingival recession.18
- STRATAGRAFT: Bioengineered skin substitute composed of allogeneic cultured keratinocytes and fibroblasts in murine collagen matrix. It is used for the treatment of adults with thermal burns containing intact dermal elements.19
Emerging fibroblast-based therapies for skin conditions
Emerging fibroblast-based therapies are showing promise in regenerative medicine, particularly for skin aging and wound healing. Innovative approaches include the use of mechanically reprogrammed fibroblasts to enhance tissue regeneration and wound healing in aged tissues, demonstrating increased extracellular matrix protein synthesis and improved tissue remodeling.20 Additionally, exosomes derived from three-dimensional cultured human skin fibroblast spheroids have been shown to ameliorate skin photoaging by promoting collagen synthesis and reducing inflammation.21 Allogeneic dermal progenitor fibroblasts have also been used in clinical settings, offering versatile applications in cutaneous regenerative medicine.22 Furthermore, engineered extracellular vesicles from skin fibroblasts are being explored to drive direct reprogramming into endothelial cells, enhancing wound closure and vascularization without the need for viral vectors.23 These advancements highlight the potential of dermal fibroblast-based therapies to address various skin disorders and improve skin health.New horizons in fibroblast research
Current research explores the role of fibroblasts in autoimmune and inflammatory skin conditions.24,25 Understanding how fibroblasts contribute to the pathogenesis of conditions such as systemic sclerosis and lupus can lead to the development of fibroblast-targeted therapies to modulate immune responses. Researchers are also exploring the use of HLA-typed and disease-specific fibroblasts to develop personalized treatments for various conditions, including autoimmune diseases, inflammatory disorders, and genetic skin diseases.26,27 Moreover, researchers are investigating the role of fibroblasts in skin microbiome interactions and how fibroblasts communicate with and influence the skin microbiome.28 These studies can lead to the development of probiotic approaches that leverage fibroblast-microbiome interactions. Studies are also ongoing to investigate the role of fibroblasts in cellular senescence to target the root causes of skin aging at the molecular level.29Challenges in skin fibroblast research
Despite significant progress in fibroblast research, researchers face several challenges (Figure 3). The limited availability of tissue- and disease-specific fibroblasts due to difficulties in obtaining fibroblasts from specific anatomical locations or from patients with rare skin conditions makes it challenging to develop accurate disease models.30 In addition, fibroblasts can undergo phenotypic changes during long-term culture, potentially affecting experimental outcomes.31 There is a need for better methods to isolate and expand specialized fibroblast populations and for improved culture systems that better mimic the in vivo environment. Furthermore, inconsistencies in serum or fetal bovine serum (FBS) used in culture media might affect the reproducible growth and function of fibroblasts.32 In addition, batch-to-batch variations in animal-derived media components can lead to poor reproducibility of results.33 Check out our media & reagents specifications guide to have a clear understanding on how to define the specifications of our cell culture media.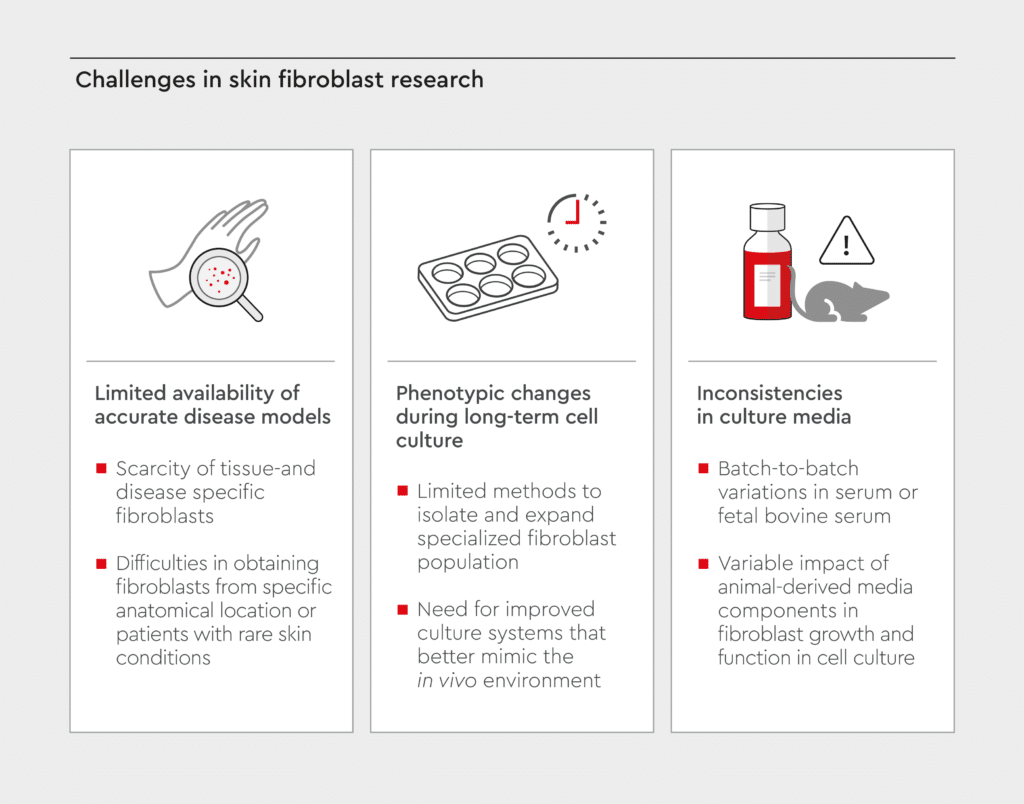
Figure 3: Challenges in skin fibroblast research.
Visual overview of the main challenges faced in skin fibroblast research.
Innovation in skin fibroblast culture: our new defined and animal component-free medium
To address the challenges of variability and reproducibility in fibroblast research, we are launching Fibroblast Growth Medium D-ACF, a defined and animal component-free (D-ACF) medium for fibroblasts. This innovative solution offers the following advantages:- Consistent cell growth and function
- Elimination of serum-related variability
- Enhanced experimental reproducibility
- Standardized culture conditions
- Defined composition: Defined components and batch-to-batch consistency, allowing for better control of the culture environment.
- Animal component-free: Reduces ethical concerns associated with the use of animal-derived products and minimizes variability.
- Enhanced performance: Optimal growth kinetics, maintained cellular phenotype, and improved reproducibility in our Normal Human Dermal Fibroblasts (NHDF) from juvenile donors.
Figure 4: Our new latest offer for animal component free dermatology research.
Our Fibroblast Growth Medium D-ACF enables standardized culture of fibroblasts and reduces the risk of cross-contamination, enhancing research translation and ensuring reproducible results without the additional cost of media optimization.
References
Expand
-
- Stunova A, Vistejnova L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018;39:137-150. doi:10.1016/j.cytogfr.2018.01.003
- Plikus MV, Wang X, Sinha S, et al. Fibroblasts: Origins, definitions, and functions in health and disease. Cell. 2021;184(15):3852-3872. doi:10.1016/j.cell.2021.06.024
- Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5(3):119-136. doi:10.1089/wound.2014.0561
- Boraldi F, Lofaro FD, Bonacorsi S, Mazzilli A, Garcia-Fernandez M, Quaglino D. The role of fibroblasts in skin homeostasis and repair. Biomedicines. 2024;12(7):1586. doi:10.3390/biomedicines12071586
- Knoedler S, Broichhausen S, Guo R, et al. Fibroblasts – the cellular choreographers of wound healing. Front Immunol. 2023;14. doi:10.3389/fimmu.2023.1233800
- Parker JB, Valencia C, Akras D, et al. Understanding fibroblast heterogeneity in form and function. Biomedicines. 2023;11(8):2264. doi:10.3390/biomedicines11082264
- Foote AG, Wang Z, Kendziorski C, Thibeault SL. Tissue specific human fibroblast differential expression based on RNAsequencing analysis. BMC Genomics. 2019;20(1):308. doi:10.1186/s12864-019-5682-5
- Ospelt C, Frank-Bertoncelj M. Why location matters — site-specific factors in rheumatic diseases. Nat Rev Rheumatol. 2017;13(7):433-442. doi:10.1038/nrrheum.2017.96
- Madsen SF, Sand JMB, Juhl P, et al. Fibroblasts are not just fibroblasts: clear differences between dermal and pulmonary fibroblasts’ response to fibrotic growth factors. Sci Rep. 2023;13(1):9411. doi:10.1038/s41598-023-36416-6
- Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25(8):599-616. doi:10.1038/s41580-024-00715-1
- Cen R, Wang L, He Y, et al. Dermal fibroblast migration and proliferation upon wounding or lipopolysaccharide exposure is mediated by stathmin. Front Pharmacol. 2022;12. doi:10.3389/fphar.2021.781282
- Castaño O, Pérez-Amodio S, Navarro-Requena C, Mateos-Timoneda MÁ, Engel E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv Drug Deliv Rev. 2018;129:95-117. doi:10.1016/j.addr.2018.03.012
- Bayer IS. Advances in fibrin-based materials in wound repair: A review. Molecules. 2022;27(14):4504. doi:10.3390/molecules27144504
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314-321. doi:10.1038/nature07039
- Fernandez-Guarino M, Naharro-Rodriguez J, Bacci S. Aberrances of the wound healing process: A review. Cosmetics. 2024;11(6):209. doi:10.3390/cosmetics11060209
- Zhao R, Jackson CJ, Xue M. Extracellular matrix and other factors that impact on cutaneous scarring. In: Shiffman MA, Low M, eds. Chronic Wounds, Wound Dressings and Wound Healing. Springer International Publishing; 2021:135-178. doi:10.1007/15695_2018_132
- Sinha S, Sparks HD, Labit E, et al. Fibroblast inflammatory priming determines regenerative versus fibrotic skin repair in reindeer. Cell. 2022;185(25):4717-4736.e25. doi:10.1016/j.cell.2022.11.004
- Rahnama M, Ghasemzadeh N, Ebrahimi Y, Golchin A. A comprehensive evaluation of dermal fibroblast therapy in clinical trials for treating skin disorders and cosmetic applications: a scoping review. Stem Cell Res Ther. 2024;15(1):318. doi:10.1186/s13287-024-03892-0
- STRATAGRAFT. FDA. January 31, 2024. Accessed December 9, 2024. https://www.fda.gov/vaccines-blood-biologics/stratagraft
- Roy B, Pekec T, Yuan L, Shivashankar GV. Implanting mechanically reprogrammed fibroblasts for aged tissue regeneration and wound healing. Aging Cell. 2024;23(2):e14032. doi:10.1111/acel.14032
- Hu S, Li Z, Cores J, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13(10):11273-11282. doi:10.1021/acsnano.9b04384
- Laurent A, Rey M, Scaletta C, et al. Retrospectives on three decades of safe clinical experience with allogeneic dermal progenitor fibroblasts: high versatility in topical cytotherapeutic care. Pharmaceutics. 2023;15(1):184. doi:10.3390/pharmaceutics15010184
- Rincon‐Benavides MA, Mendonca NC, Cuellar‐Gaviria TZ, et al. Engineered vasculogenic extracellular vesicles drive nonviral direct conversions of human dermal fibroblasts into Induced endothelial cells and improve wound closure. Adv Ther. 2023;6(3):2200197. doi:10.1002/adtp.202200197
- Chen X, Wu Y, Jia S, Zhao M. Fibroblast: a novel target for autoimmune and inflammatory skin diseases therapeutics. Clin Rev Allergy Immunol. 2024;66(3):274-293. doi:10.1007/s12016-024-08997-1
- He Y, Han Z, Zhang Q, Liu L, Chang J. Role of fibroblasts in nonfibrotic autoimmune skin diseases. Mol Med. 2024;30(1):178. doi:10.1186/s10020-024-00949-x
- D’Antonio M, Reyna J, Jakubosky D, et al. Systematic genetic analysis of the MHC region reveals mechanistic underpinnings of HLA type associations with disease. McCarthy MI, Dendrou C, Cheung VG, Cordell H, Meyer D, eds. eLife. 2019;8:e48476. doi:10.7554/eLife.48476
- Kim Y, Park N, Rim YA, et al. Establishment of a complex skin structure via layered co-culture of keratinocytes and fibroblasts derived from induced pluripotent stem cells. Stem Cell Res Ther. 2018;9(1):217. doi:10.1186/s13287-018-0958-2
- SanMiguel A, Grice EA. Interactions between host factors and the skin microbiome. Cell Mol Life Sci. 2015;72(8):1499-1515. doi:10.1007/s00018-014-1812-z
- Lee JH, Park J, Shin DW. The molecular mechanism of polyphenols with anti-aging activity in aged human dermal fibroblasts. Molecules. 2022;27(14):4351. doi:10.3390/molecules27144351
- Lynch MD, Watt FM. Fibroblast heterogeneity: implications for human disease. J Clin Invest. 2018;128(1):26-35. doi:10.1172/JCI93555
- Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev. 1993;73(3):617-638. doi:10.1152/physrev.1993.73.3.617
- van der Valk J, Brunner D, De Smet K, et al. Optimization of chemically defined cell culture media – Replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010;24(4):1053-1063. doi:10.1016/j.tiv.2010.03.016
- Zhou Y, Richards AM, Wang P. Characterization and Standardization of Cultured Cardiac Fibroblasts for Ex Vivo Models of Heart Fibrosis and Heart Ischemia. Tissue Eng Part C Methods. 2017;23(7):422-433. doi:10.1089/ten.tec.2017.0169
Contact our experts
Are you working to advance research on dermatology and regenerative medicine? Our specialists are ready to support your journey. Contact our team today to learn how our new Fibroblast Growth Medium D-ACF can help enhance your research and optimize experimental outcomes. Let’s work together to unlock the full potential of skin fibroblast research.
Contact us
