Endothelial to mesenchymal transition (EndMT) is a complex biological process that is essential for various physiological functions, from embryonic development to tissue repair.1 But what happens when the mechanisms underlying EndMT are deregulated? In this article, we explore the intricacies of EndMT, discussing its mechanisms, role in health and disease, and therapeutic potential.
What is endothelial to mesenchymal transition?
Endothelial to mesenchymal transition is a complex cellular process involving endothelial cells, which are the building blocks of blood vessels. During this process, endothelial cells undergo a phenotypic switch, transforming into mesenchymal cells (Figure 1).1 Subsequently, mesenchymal cells can migrate away from the endothelium and differentiate into various cell types. Numerous signaling pathways and gene expression changes mediate this transformation in cell structure and behavior.2 Endothelial cells undergoing EndMT lose their endothelial markers, such as vascular endothelial cadherin (VE-cadherin) and CD31, and acquire mesenchymal markers like vimentin, fibroblast-specific protein 1 (FSP1), α-smooth muscle actin (α-SMA), and fibronectin.3 Transcription factors like Snail, Slug, and Twist drive this shift in gene expression.2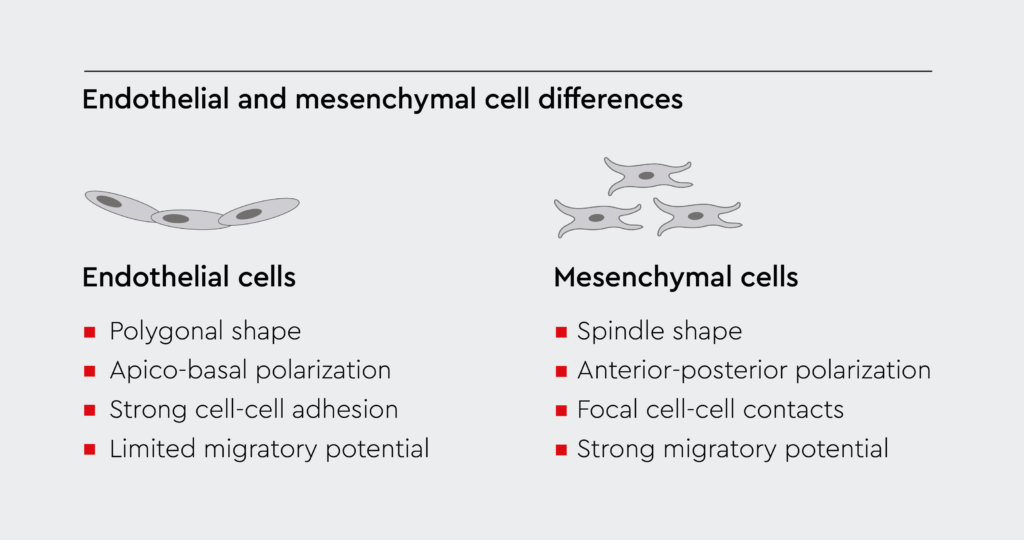
Figure 1a: Endothelial cells vs. mesenchymal cells.
Endothelial cells are different from mesenchymal cells. During the process of EndMT, endothelial cells take on the typical morphology and function of mesenchymal cells.
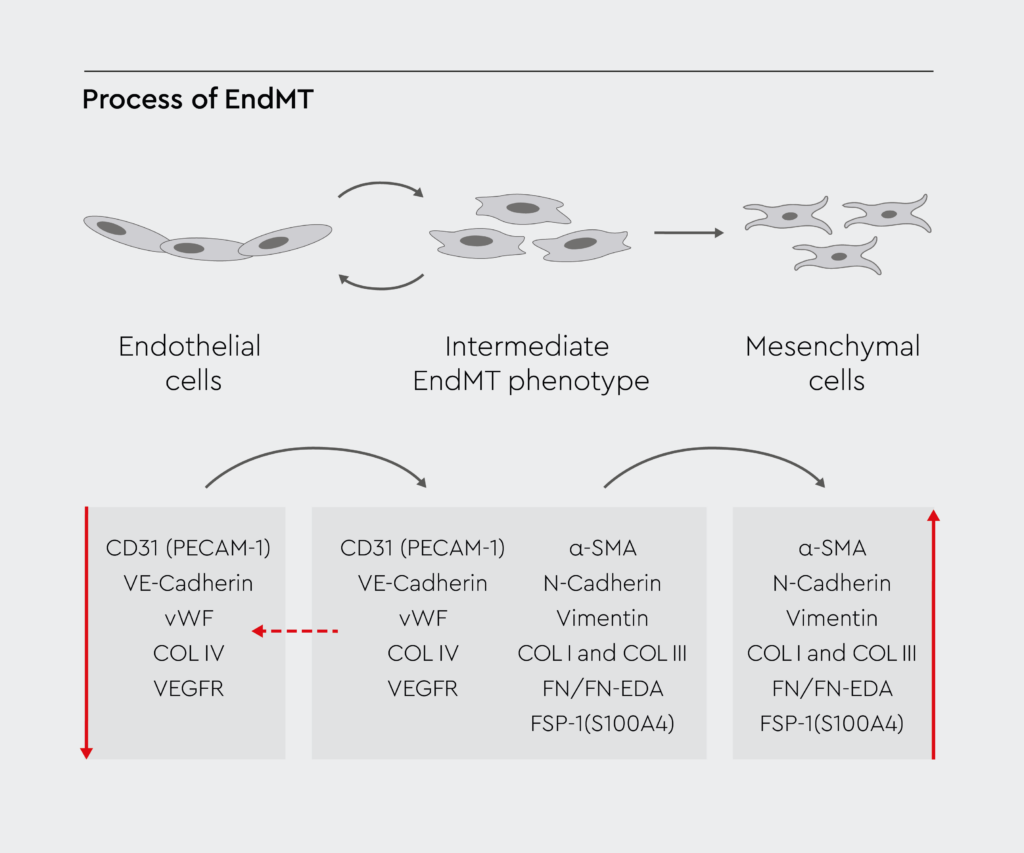
Figure 1b: The process of EndMT.
The diagram illustrates the molecular mechanisms regulating the transformation of endothelial cells to mesenchymal cells. Redrawn from Piera-Velazquez et al., 2019.1
Molecular players of endothelial to mesenchymal transition
Endothelial to mesenchymal transition has important implications for health and disease. Therefore, various proteins and signaling pathways work together to ensure it occurs only when and where necessary.4 Key signaling pathways regulating EndMT include the following:- Bone morphogenetic proteins (BMPs) promote the expression of proinflammatory cytokines that induce EndMT when the structure and function of vascular endothelial cells are abnormal.5 BMP signaling also enhances the heterogeneity of endothelial cells, which is essential for the transition.
- Transforming growth factor-beta (TGF-β) signaling induces the expression of transcription factors like Snail and Slug, which drive the transition from endothelial to mesenchymal phenotype.6
- Wnt/β-catenin signaling activates the transcription of target genes involved in the process.7
- Notch signaling induces the expression of mesenchymal markers and promotes EndMT.8
- Tumor necrosis factor-alpha (TNF-α) is a proinflammatory cytokine that can induce EndMT by downregulating endothelial receptors.9
Multifaceted roles of EndMT: A double-edged sword
EndMT plays crucial roles in embryonic development, tissue repair, and vascular homeostasis. However, when dysregulated, it can contribute to the development or progression of various pathological conditions.1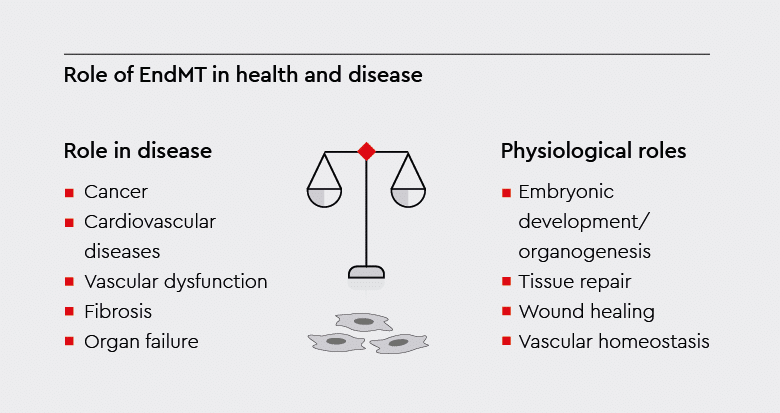
Fig. 2: Role of EndMT in health and disease.
The flowchart illustrates physiological processes and pathological conditions associated with EndMT.
Physiological functions of endothelial to mesenchymal transition
Under physiological conditions, EndMT regulates various biological processes that are essential for development and tissue homeostasis.- Embryonic development: During embryogenesis, it facilitates the delamination of endothelial cells from the atrioventricular canal. This allows their migration and subsequent differentiation into various cell lineages, essential for the development of the valves and septa of the heart.3
- Tissue repair: In response to injury, it contributes to the formation of fibroblast-like cells and the production of extracellular matrix (ECM) proteins. The process is essential for wound healing and tissue repair.11
- Vascular homeostasis: EndMT contributes to vascular maintenance and plasticity, ensuring proper endothelial function and vessel integrity. Furthermore, it plays a role in vascular remodeling during angiogenesis, facilitating the adaptation of blood vessels to changing physiological conditions.12,13
Role of endothelial to mesenchymal transition under pathological conditions
Excessive or inappropriate transformation of endothelial cells to mesenchymal cells can lead to pathological conditions, such as fibrosis and cancer. Pathological conditions associated with EndMT include the following:- Cancer: Mesenchymal-like cells derived from endothelial cells exhibit enhanced migratory and invasive properties. The acquisition of these properties facilitates their extravasation into surrounding tissues and distant organs. These migrating cells contribute to the formation of pre-metastatic niches, fostering the colonization and outgrowth of metastatic tumor cells.14,15
- Cardiovascular diseases: Transformation of endothelial cells to mesenchymal-like cells contributes to cardiac fibrosis and endothelial dysfunction, causing vascular stiffness and narrowing. In turn, vascular stiffness can lead to atherosclerosis, diabetes, myocardial infarction, and heart failure.1
- Fibrosis: In response to chronic injury or inflammation, endothelial cells undergo EndMT, contributing to excessive deposition of ECM proteins and the development of tissue fibrosis.1
- Organ failure: EndMT-derived myofibroblasts perpetuate fibrogenesis through the secretion of profibrotic factors and the recruitment of immune cells, which could lead to organ dysfunction and failure.16
Taking EndMT from bench to bedside
EndMT is an attractive therapeutic target because of its important role in health and disease.17 Recent advances in our understanding of the molecular mechanisms underlying EndMT have opened new avenues for therapeutic intervention.Progress in targeting EndMT
Targeting key regulators of endothelial to mesenchymal transition, such as TGF-β and Notch signaling, holds promise for mitigating disease progression and improving patient outcomes.17 Recent efforts to potentially treat diseases by targeting EndMT include:- Anticancer therapy: Recent findings have shown that EndMT may contribute to resistance to cancer therapies, such as chemotherapy, antiangiogenic therapy, and radiation therapy.18 Therefore, combination strategies involving its inhibition may enhance the efficacy of cancer treatments.
- Atherosclerosis treatment: Researchers have found that vascular endothelial cells are transformed into mesenchymal stem cells (MSCs) through EndMT in fibrodysplasia ossificans progressiva, in which acute inflammation leads to ectopic bone formation. This finding opens up new possibilities for the treatment of atherosclerosis by targeting MSCs derived from endothelial cells.19
- Tissue engineering and regeneration: Emerging results link EndMT to the development and progression of fibrotic disease. Moreover, recent reports have emphasized its potential in tissue engineering and regenerative applications as an alternative to stem cell therapies.20
Challenges in studying endothelial to mesenchymal transition
Developing targeted EndMT inhibitors requires an improved understanding of the molecular mechanisms regulating this process in different disease contexts.- In vivo research: Animal models have been used to obtain insights into EndMT function in vivo. For example, researchers have used cell lineage tracing to track this process in mice after myocardial infarction.21 Zebrafish models have also been used to visualize the transition during cardiac valve formation.22 Although useful, animal models do not accurately represent the mechanisms underlying the switch from endothelial cells to mesenchymal-like cells in humans.
- In vitro research: In vitro, EndMT can be stimulated in endothelial cell cultures (e.g., with TGF-β), enabling mechanistic studies.23 In addition, coculturing endothelial cells with cancer cell types captures microenvironmental conversion regulation.24 However, studying this transformation in vitro poses several challenges, including the complexity of its regulatory networks, the lack of standardized experimental models, and the dynamic nature of endothelial plasticity. It requires interdisciplinary collaboration and innovative research methodologies to address these challenges.
Innovative solutions for studying EndMT in vitro
As cell culture experts, we provide a comprehensive portfolio of human endothelial cells from various tissues and specialized media tailored for EndMT-research:- Human Umbilical Vein Endothelial Cells (HUVEC) are derived from the endothelium of veins from the umbilical cord. They are commonly used as models in studies related to cell adhesion, cell migration, angiogenesis, and inflammation.
- Human Umbilical Artery EndothelialCells (HUAEC) are isolated from the endothelium of arteries in the umbilical cord. They can be used as models in vascular biology research, including EndMT studies.
- Human Pulmonary Microvascular Endothelial Cells (HPMEC) are derived from the lung microvasculature and are used in studies related to lung diseases, inflammation, and EndMT.
- Human Cardiac Microvascular Endothelial Cells (HCMEC) are isolated from the microvasculature of the heart. They are used in cardiovascular research, including studies on heart diseases and EndMT.
- Human Coronary Artery Endothelial Cells (HCAEC) are derived from the coronary artery endothelium of single donors.
- Human Aortic Endothelial Cells (HAOEC) are isolated from the aorta and used in vascular biology research, including studies on aortic diseases and EndMT.
- Endothelial Cell Growth Media: We offer a wide range of media that are optimized for endothelial cells, including Endothelial Cell Growth Medium, Endothelial Cell Growth Medium 2, Endothelial Cell Growth Medium MV, and Endothelial Cell Growth Medium MV2. These media provide the nutrients necessary for the growth and maintenance of endothelial cells in vitro, ensuring robust and reproducible results.
References
Expand
- Piera-Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: Role in physiology and in the pathogenesis of human diseases. Physiol Rev. 2019;99(2):1281-1324. doi:10.1152/physrev.00021.2018
- Pinto MT, Ferreira Melo FU, Malta TM, et al. Endothelial cells from different anatomical origin have distinct responses during SNAIL/TGF-β2-mediated endothelial-mesenchymal transition. Am J Transl Res. 2018;10(12):4065-4081.
- Medici D, Kalluri R. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol. 2012;22(5-6):379-384. doi:10.1016/j.semcancer.2012.04.004
- Kovacic JC, Dimmeler S, Harvey RP, et al. Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(2):190-209. doi:https://doi.org/10.1016/j.jacc.2018.09.089
- Niu Z, Su G, Li T, et al. Vascular calcification: New insights into BMP type I receptor A. Front Pharmacol. 2022;13:887253. doi:10.3389/fphar.2022.887253
- Ma J, van der Zon G, Gonçalves MAF V, et al. TGF-β-induced endothelial to mesenchymal transition is determined by a balance between SNAIL and ID factors. Front cell Dev Biol. 2021;9:616610. doi:10.3389/fcell.2021.616610
- Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4(4):469-483. doi:10.1242/dmm.006510
- Lin Q-Q, Zhao J, Zheng C-G, Chun J. Roles of notch signaling pathway and endothelial-mesenchymal transition in vascular endothelial dysfunction and atherosclerosis. Eur Rev Med Pharmacol Sci. 2018;22(19):6485-6491. doi:10.26355/eurrev_201810_16062
- Adjuto-Saccone M, Soubeyran P, Garcia J, et al. TNF-α induces endothelial-mesenchymal transition promoting stromal development of pancreatic adenocarcinoma. Cell Death Dis. 2021;12(7):649. doi:10.1038/s41419-021-03920-4
- Anbara T, Sharifi M, Aboutaleb N. Endothelial to mesenchymal transition in the cardiogenesis and cardiovascular diseases. Curr Cardiol Rev. 2020;16(4):306-314. doi:10.2174/1573403X15666190808100336
- Guo L, Mi J, Zhang H, et al. Endothelial–mesenchymal transition as a novel mechanism for generating myofibroblasts during wound healing and scarring. J Cosmet Dermatol. 2023;22(2):661-668. doi:https://doi.org/10.1111/jocd.15466
- Alvandi Z, Bischoff J. Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41(9):2357-2369. doi:10.1161/ATVBAHA.121.313788
- Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun. 2017;8(1):14361. doi:10.1038/ncomms14361
- Gasparics Á, Rosivall L, Krizbai IA, Sebe A. When the endothelium scores an own goal: endothelial cells actively augment metastatic extravasation through endothelial-mesenchymal transition. Am J Physiol Heart Circ Physiol. 2016;310(9):H1055-63. doi:10.1152/ajpheart.00042.2016
- Krizbai IA, Gasparics Á, Nagyőszi P, et al. Endothelial-mesenchymal transition of brain endothelial cells: possible role during metastatic extravasation. PLoS One. 2015;10(3):e0119655. doi:10.1371/journal.pone.0119655
- Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci. 2017;18(10). doi:10.3390/ijms18102157
- Hulshoff MS, Xu X, Krenning G, Zeisberg EM. Epigenetic regulation of endothelial-to-mesenchymal transition in chronic heart disease. Arterioscler Thromb Vasc Biol. 2018;38(9):1986-1996. doi:10.1161/ATVBAHA.118.311276
- Yin Z, Wang L. Endothelial-to-mesenchymal transition in tumour progression and its potential roles in tumour therapy. Ann Med. 2023;55(1):1058-1069. doi:10.1080/07853890.2023.2180155
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16(12):1400-1406. doi:10.1038/nm.2252
- Medici D. Endothelial-mesenchymal transition in regenerative medicine. Stem Cells Int. 2016;2016:6962801. doi:10.1155/2016/6962801
- Tombor LS, John D, Glaser SF, et al. Single cell sequencing reveals endothelial plasticity with transient mesenchymal activation after myocardial infarction. Nat Commun. 2021;12(1):681.
- O’Donnell A, Yutzey KE. To endoMT or not to endoMT: zebrafish heart valve development. Circ Res. 2020;126(8):985-987. doi:10.1161/CIRCRESAHA.120.316846
- Van Meeteren LA, Ten Dijke P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2012;347(1):177-186. doi:10.1007/s00441-011-1222-6
- Sun X, Fang J, Ye F, et al. Diffuse large B-cell lymphoma promotes endothelial-to-mesenchymal transition via WNT10a/beta-catenin/Snail signaling. Front Oncol. 2022;12:871788. doi:10.3389/fonc.2022.871788
Contact our experts Reach out to our specialists to learn more about how our products can help you accelerate your research on EndMT. Let’s shape the future of EndMT research together.
