Transfer from PDX to in vitro assays
Xenograft models are extensively used to provide information about the safety and efficacy in drug development, especially for targeted cancer therapies. Patient-derived xenograft (PDX) mice are the preferred mouse tumor models, but cell line-derived xenografts (CDXs) and nude rat xenografts are also used. Nevertheless, still too many drugs fail in clinic that seemed promising in such models. More predictive data are needed to increase optimal translation into clinic.
Further analysis of the PDX tumor material ex vivo is crucial for the follow-up of PDX mouse studies. Be it xenograft-derived primary cell lines for screening purpose or advanced 3D tumor models for detailed characterization. The advantages are clear: re-evaluate drug efficacy, re-prioritize lead molecule series, and understand why the drug candidate may have not performed in the PDX mouse to ensure a solid data set before moving to clinical trials.
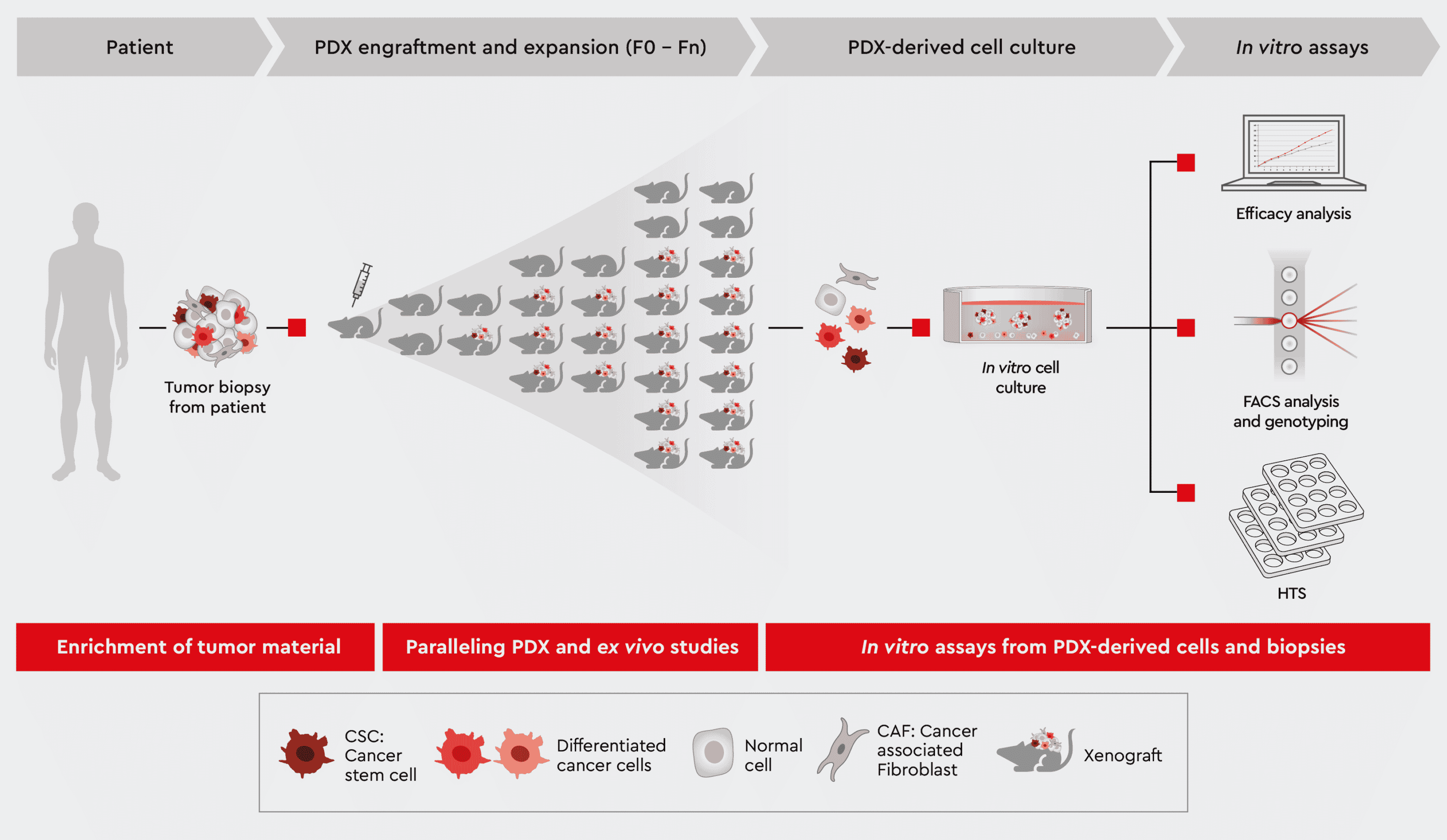
Figure 1: Overview of Patient-Derived Xenograft (PDX) model approach. Exploit the following sections for further information how we help you leveraging your PDX mouse project.
We support you from
- Enrichment of high-quality tumor cells for transplantation into PDX mice,
- Parallel employment of in vitro studies using the same patient-derived biopsy,
- Ex vivo expansion for post PDX in vitro assay panels.
Ex vivo data from PDX mice-derived cell cultures are physiologically more relevant than data from established cell lines, more cost-effective and faster than in vivo studies. Therefore, they allow more extensive testing of the lead molecule and other series members.
Let’s look at a typical scenario: few candidates can only be submitted to PDX mouse studies. And if then the lead molecule did not perform well in the study, users must cautiously select follow-up candidates. Conducting ex vivo studies with cell materials from the same PDX mouse is now the best option for data comparability and addressing specific questions. For example, which properties of the drug candidates need to be improved to warrant success? Ex vivo studies are an elegant way to rescue a lead series before starting the next PDX mouse test. However, suppose ex vivo cell cultures deviate too far from the original tumor situation.
Expand PDX-cells for ex vivo assays
A convenient way to expand the PDX mouse for in vitro assays
We’ve developed a media toolbox that allows users to establish 2D and 3D tumor models ex vivo without losing the original tumor’s traits. The toolbox works by conserving cancer stem cells which were responsible for the original tumor initiation and formation within the individual patient.
The design of our media toolbox allows to:
- Perform ex vivo assays directly from PDX mouse-derived tumor models
- Establish primary cell lines from this model that allow extensive testing and screening, but also to create master stocks for later studies
- Develop 3D tumor models from spheroids to organoids to address more complicated tumor biology questions
Yet another advantage of the media: PDX model-derived tumor grafts contain mouse cancer cells, mouse fibroblasts etc. Our media eliminate mouse cells and allow only human cells to grow in the culture.
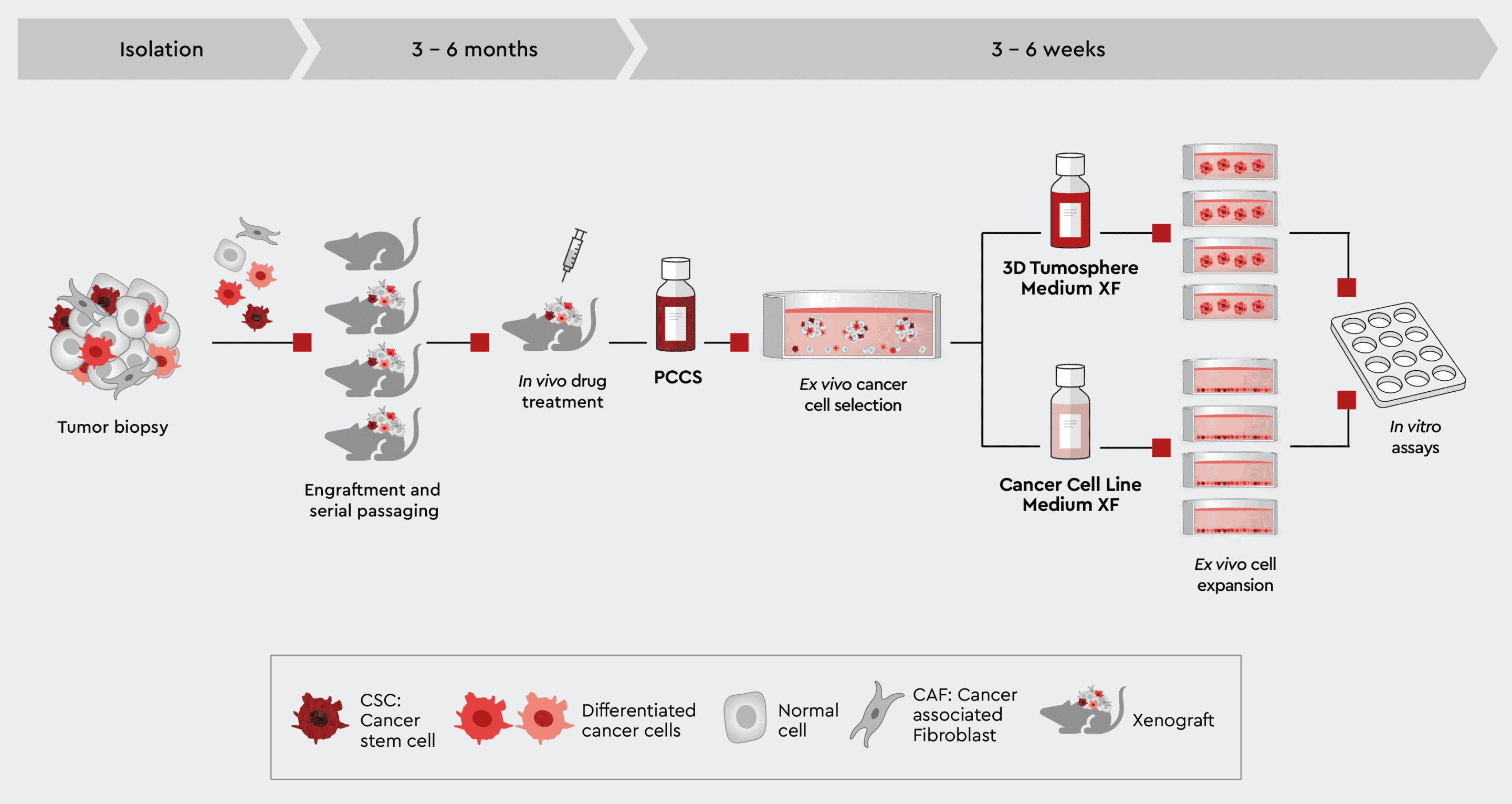
Figure 2: Establish PDX-derived 2D and 3D in vitro tumor models. Expanding your PDX-derived cells in vitro in our cancer media allows you to perform high-throughput screenings, secondary assays or even organoid models while conserving the tumor cell characteristics.
Now, the availability of a media toolbox compatible with many different tumor types that conserves tumor biology empowers users to leverage PDX mice for a complete ex vivo study panel, generating relevant data for better decision-making.
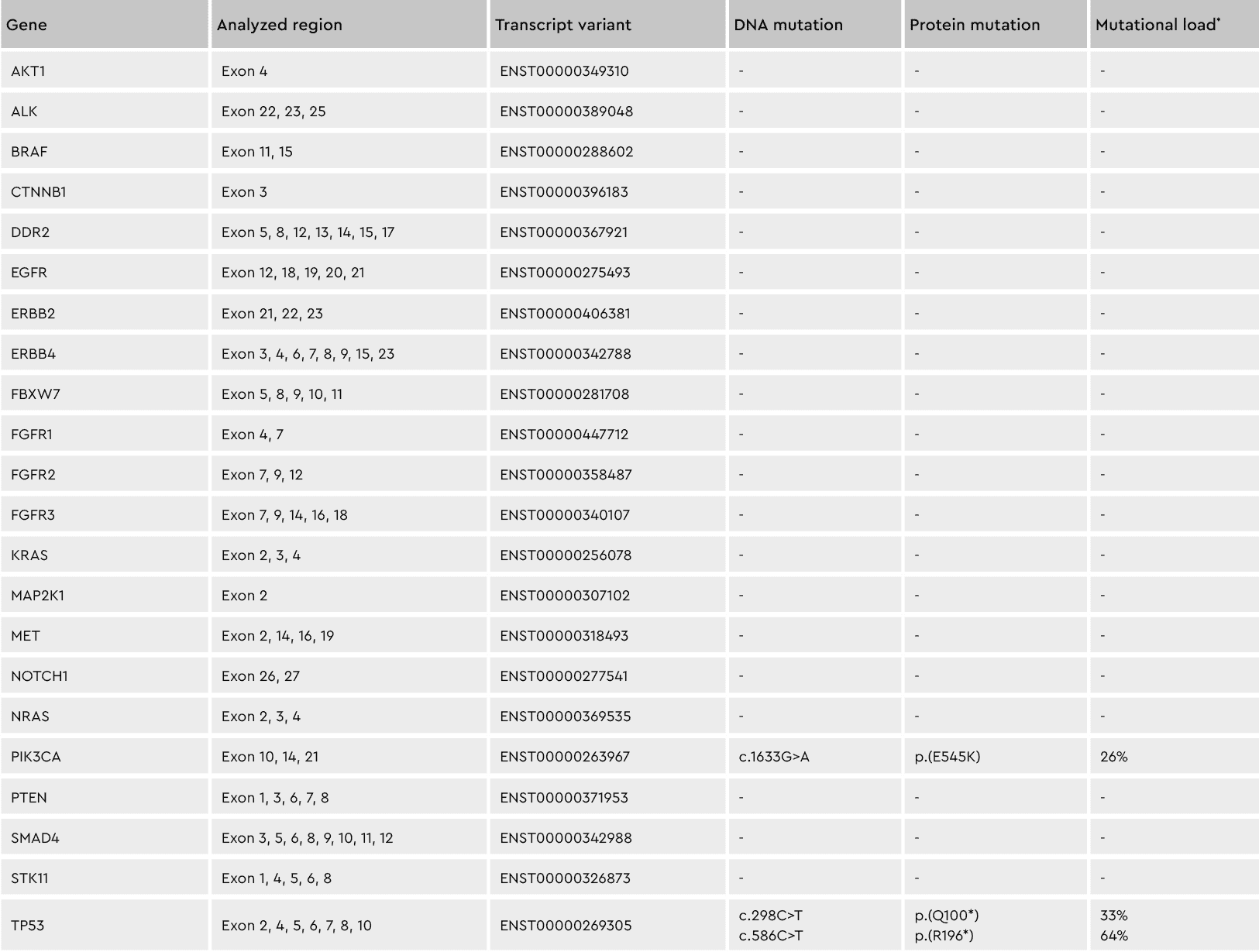
Figure 3: Conserving tumor biology over time. The genotype of cell subpopulations in our cancer media is conserved throughout the isolation and culturing process, shown by the differing percentages of the individual mutations. *Mutation load = percentage of mutated transcripts / total transcripts of each variant
Better starting material for PDX
Enriching tumor material for transplantation while conserving original tumor biology
Current protocols for generating PDX mouse models try to get fresh, patient-derived tumor material into the mouse as quickly as possible. The hope is to conserve as much of the original human tumor’s traits, architecture, and microenvironment as possible. Realistically, there’s not always sufficient material to extract enough representative tumor pieces. Furthermore, the original tumor environment may become influenced, and through successive generations, may be controlled by the mouse. For example, mouse fibroblasts differ substantially from human fibroblasts, affecting the tumor microenvironment.
Another issue is the variable success rate of generating PDX mice. This depends on the tumor type and state of malignancy. Part of the underlying requirements of a good tumor biopsy piece is the presence of cancer stem cells. Suppose a tumor sample does not contain enough (healthy) CSCs. In that case, it’s unlikely to induce tumor formation in a PDX model. And unfortunately, the tumor driving CSCs are not evenly spread throughout the tumor. Maintaining tumor traits and increasing success rates in PDX models depends on well-defined tumor cell material containing a relevant proportion of CSCs.
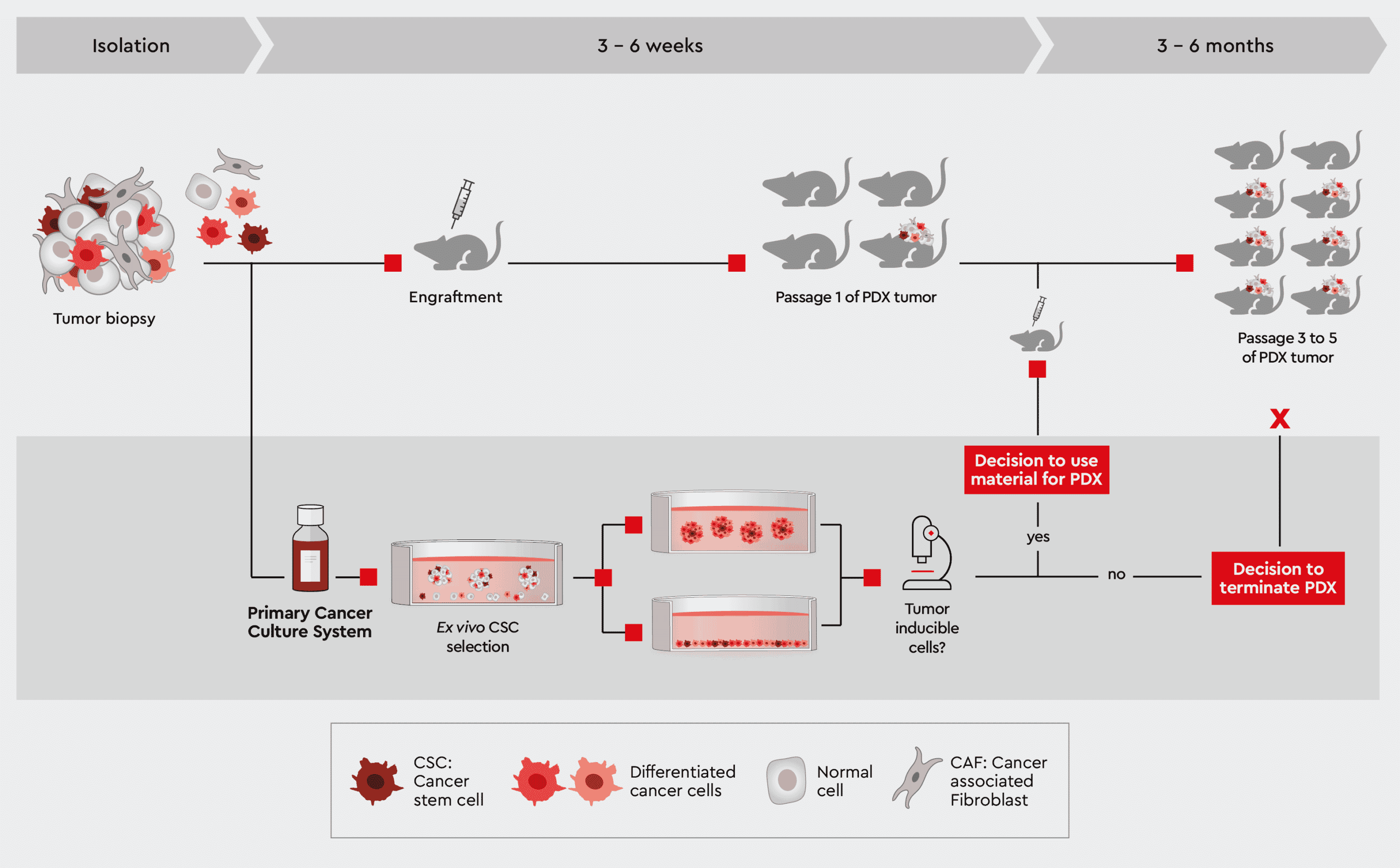
Figure 4: Characterization of patient-derived tumor material to monitor success of PDX mouse. Establishing a primary cell culture from the same biopsy and rapidly testing its capability to induce efficiently tumors in vitro helps in taking better decisions when to continue or halt a PDX mouse strain. Preparing a cell suspension from this primary cell culture enables the use of well-defined starting material for the PDX mouse.
Our cancer media toolbox has been described in the workflows above to establish PDX mouse-derived cell culture. Obviously, the toolbox can do the same for patient-derived cancer cell cultures: It maintains the original tumor traits through support and enrichment of the patient specific CSCs. With this innovative media functionality, biopsy material can now be expanded without losing the patient-specific tumor biology allowing for defined starting material such as a patient-derived primary cancer cell suspension for injection into the PDX mice.
Furthermore, a parallel characterization of biopsy material selected for initiation of a PDX or other xenograft model project can help to increase the success rate for cancer types known to have a poor up-take rate in the mouse. Our media, particularly the Primary Cancer Culture System (PCCS) and 3D Tumorsphere Medium XF, can predict the likelihood of tumor initiation in the mouse by determining the presence of cancer cells capable of initiating tumor formation (see e.g., the tumorsphere formation efficiency assay. This information is key to decide on which biopsies to bring into a PDX model. Figure 4 outlines this approach. Read more about, how to process tumor biopsies with our cancer media in From Biopsy to Assay Development.
Paralleling PDX and in vitro
Better decisions by paralleling the PDX approach with ex vivo strategies
We generally advise users to establish patient-derived primary cell cultures parallel to the PDX mouse approach, because various valuable strategies unfold to make best use of the PDX mouse:
Go/ No-go decision of ongoing PDX mouse breeding: the biopsy material can be further characterized ex vivo during the time of the first passage in mouse. If the biopsy does not contain the desired features, the specific PDX mouse line can be terminated early and re-started with better biopsy material rather than waiting for months during the subsequent mouse generations.
Better decision on drug candidate selection: the biopsy material can be used to establish primary cancer cell lines to carry out drug assays in 2D and 3D cell culture systems. This helps to better optimize and prioritize the candidates of a lead series that should eventually go into the PDX mouse model for in vivo pharmacology.
Verification of PDX mouse model data: principally, the data from the parallel ex vivo studies (patient-derived) can be compared to data of the ex vivo studies of the PDX mouse-derived material to better understand the behavior of the therapeutic agent in vivo and to take better decisions for moving into first-in-man studies.
Performing sound studies in vitro with the same biopsy material in parallel to the generation of PDX mice helps choosing the best drug candidates for the in vivo pharmacological studies.
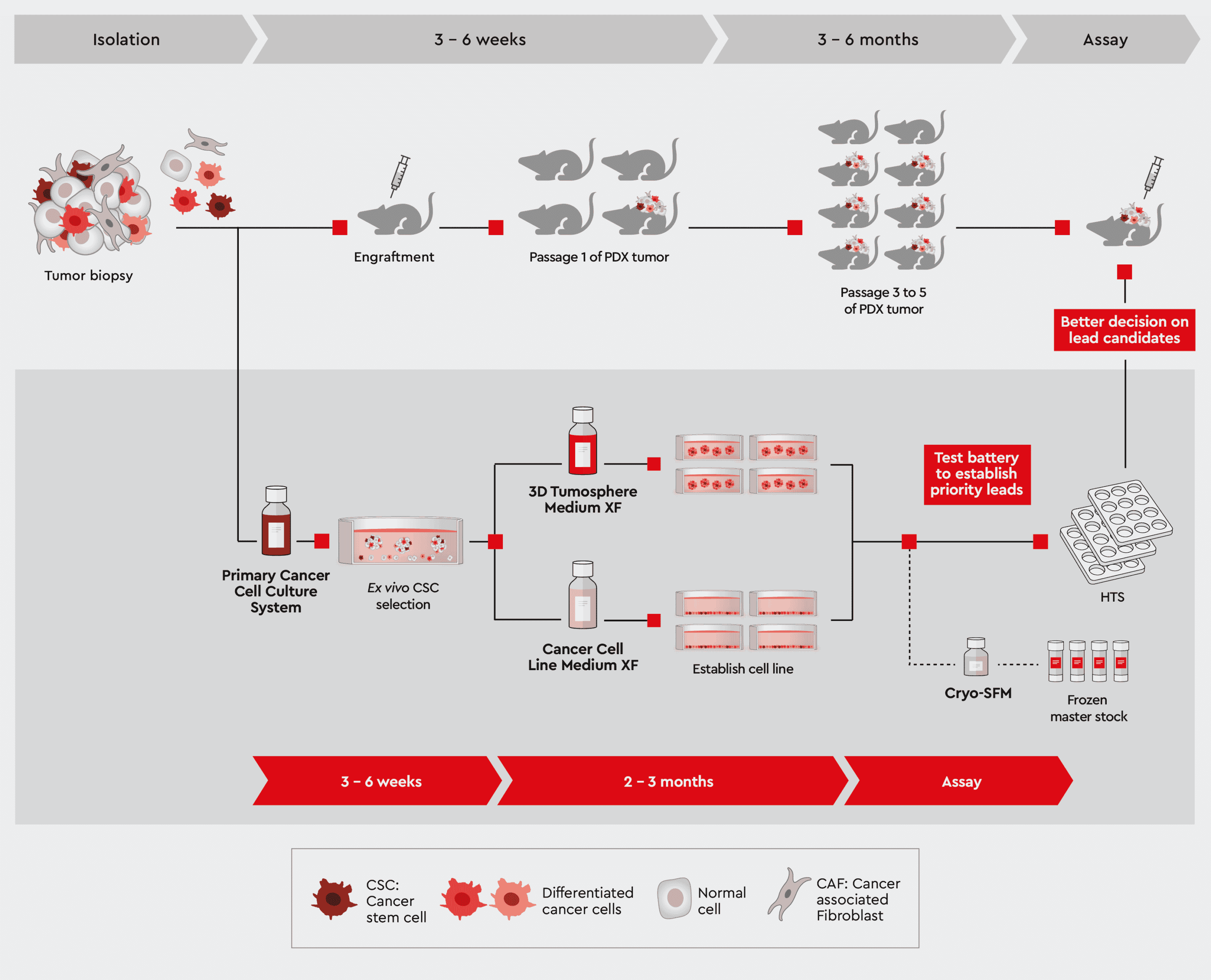
Figure 5: Parallel studies with patient-derived primary cell culture and the development of PDX models.
Get in touch with our scientists to design the appropriate ex vivo strategy.




