Did you know that one in three people have excess weight? As the global obesity epidemic continues to grow, it presents significant challenges for healthcare systems worldwide. In vitro obesity research allows for the study of adipocytes — the cells that primarily compose adipose tissue — and their interactions in controlled laboratory conditions. This provides valuable insights into overweight at the cellular and molecular levels.
The growing challenge of obesity and associated comorbidities
Obesity is a global health crisis, affecting 1 billion people worldwide.1 This complex condition is more than just excess body weight — it is a multifaceted disorder that has been associated with several chronic and potentially serious diseases.2,3 The associated comorbidities include:- Type 2 diabetes
- Cardiovascular diseases
- Endometrial, gallbladder, and esophageal cancers
- Musculoskeletal disorders
Understanding obesity in vitro
In vitro obesity research has emerged as a promising approach for studying adipose cell behavior and metabolism in a controlled environment (Figure 1). It provides molecular insights into the mechanisms of adiposity that cannot be obtained using in vivo models.7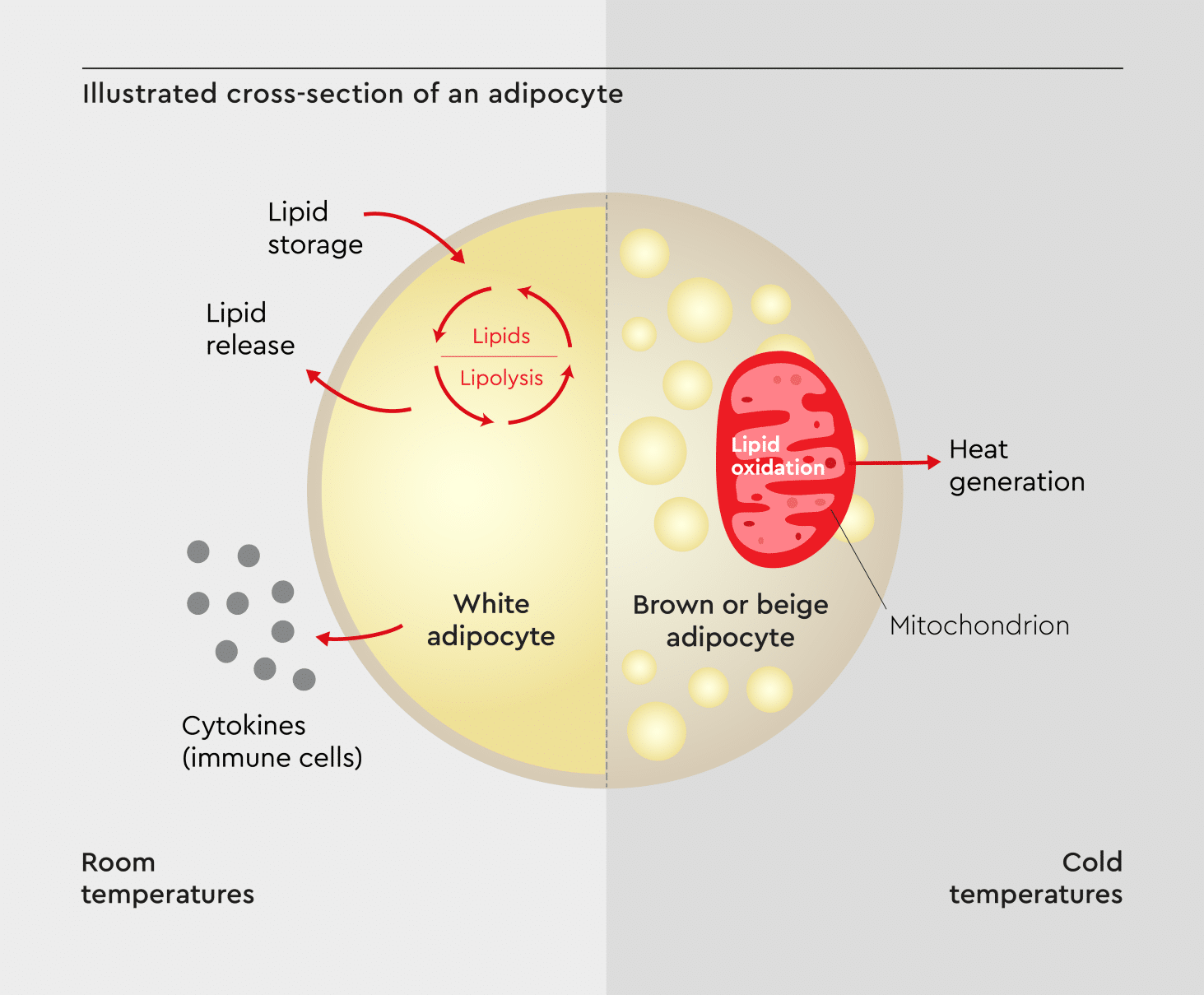
Figure 1: An illustrated cross-section of an adipocyte.
Showing key cellular components and processes (e.g., lipid droplet formation, insulin signaling, and adipokine secretion) that can be examined through in vitro obesity research. Adapted from Guilherme et al., 2019.8
Advantages of in vitro obesity research
By studying obesity in cell models, you can:- Isolate and examine specific cell types involved in fat storage and metabolism, such as white, brown, and beige adipocytes.7
- Easily study genes influencing lipid storage and metabolism.9
- Control experimental conditions, allowing for reproducible results.7
- Test potential treatments without risking human subjects or requiring animal studies.10
Key findings from in vitro obesity research
In vitro obesity research has helped improve our understanding of fat tissue and its role in health and disease:- Brown fat discovery: In vitro studies led to the identification and characterization of brown adipose tissue in adults, opening new avenues for weight loss strategies.12
- Adipokines: Research on cultured adipocytes revealed that adipose tissue is an endocrine organ, secreting hormones called adipokines that influence metabolism, inflammation, and appetite.13
- Inflammation link: In vitro studies have elucidated the role of chronic low-grade inflammation in adiposity, linking it to insulin resistance and other obesity-related complications.14
- Adipocyte differentiation: Detailed in vitro analyses have mapped out the process of adipocyte differentiation, identifying key regulators like PPARγ and C/EBPα.15
- Insulin signaling: Cell models have helped elucidate the complex insulin signaling pathways in adipocytes, providing insights into the development of insulin resistance.16
Current approaches in in vitro obesity research
The field of obesity research is evolving rapidly, with new techniques and models constantly emerging.Obesity cell culture models
Researchers are developing increasingly sophisticated in vitro models to study the behavior and metabolism of adipose cells. Some of the most promising ones include:- 3D adipose tissue models that mimic the structure of human fat tissue more accurately than traditional 2D cultures.17 They allow for a better study of cell-cell interactions, extracellular matrix influences, and tissue-level responses to stimuli.
- Co-culture systems allow researchers to study interactions between adipose cells and other cell types (e.g., immune cells and endothelial cells).18
- Microfluidic devices that simulate the dynamic environment of living tissues allow for the study of adiposity in a more physiologically relevant context.18
- Patient-derived adipocytes offer a personalized in vitro platform to study individual variations in adipose tissue function.19
Role of extracellular vesicles in obesity
Recent research has highlighted the importance of extracellular vesicles (EVs) in obesity. These microscopic particles are released by cells and act as inter-organ communicators, carrying important signals between adipose tissue and other organs.20 In vitro studies have shown that excess weight alters the composition and function of EVs released by adipocytes.21 Therefore, analyzing EVs in vitro could lead to new biomarkers for obesity-related diseases. In addition, studies have shown the role of EVs in spreading obesity-associated inflammation throughout the body (Figure 2).22,23 Adipose tissue is surrounded by immune cells, including CD8 T cells, CD4 T cells, and M2 macrophages. Adipocyte-derived and immune cell-derived EVs carry inflammatory mediators, contributing to chronic inflammation. This can lead to conditions such as insulin resistance, type 2 diabetes mellitus (T2DM), rheumatoid arthritis, and inflammatory bowel disease (IBD). Deregulated T cell subsets perpetuate inflammation, linking obesity to immune response and inflammation-related diseases.22,23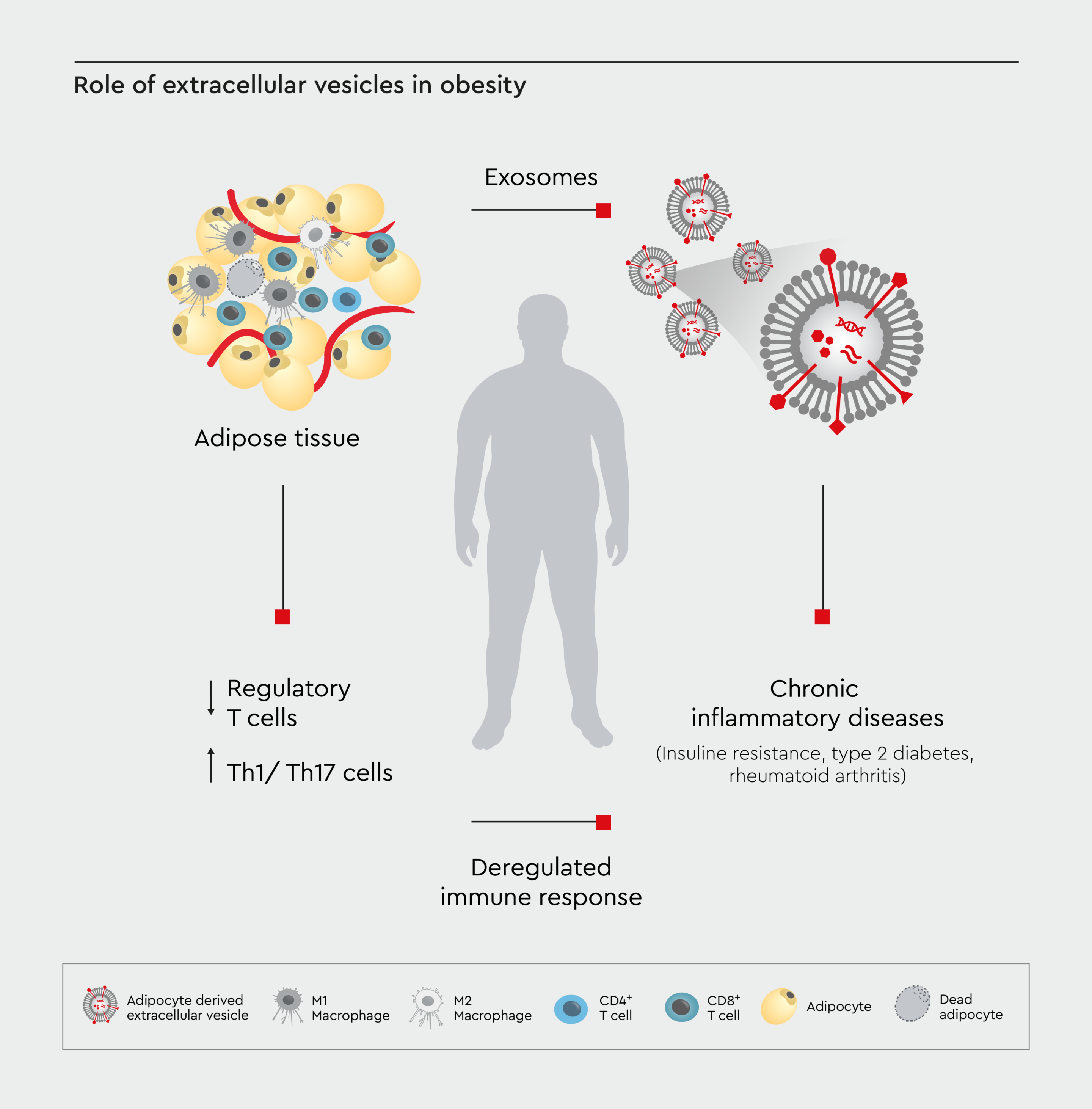
Figure 2: Role of extracellular vesicles in obesity.
A schematic diagram depicting the role of extracellular vesicles in inter-organ communication related to obesity. Adapted from Kumar et al., 2022.23
Linking in vitro findings to clinical applications
In vitro research can lead to new diagnostic tools and therapeutic targets for obesity-related diseases.17,18 Efforts to accelerate the development of weight loss treatments through in vitro studies include:- High-throughput screening of potential anti-obesity compounds.
- Personalized medicine approaches using patient-derived cells.
- Development of biomarkers for early detection of obesity-related complications and comorbidities.
Challenges in obesity research
Despite significant progress in understanding the pathophysiology of adiposity and its link to comorbidities, obesity research faces several challenges (Figure 3):- Replicating the complex in vivo environment of adipose tissue, including its interactions with other organs and systems, remains challenging.24
- Accounting for individual variability in overweight susceptibility and adipose cell behavior is difficult in in vitro models.25
- Integrating findings from different research models (in vitro, animal, and human studies) to create a comprehensive understanding of obesity is an ongoing challenge.11,26
- Studying the long-term effects of excess weight in vitro is limited by the lifespan of cell cultures.26
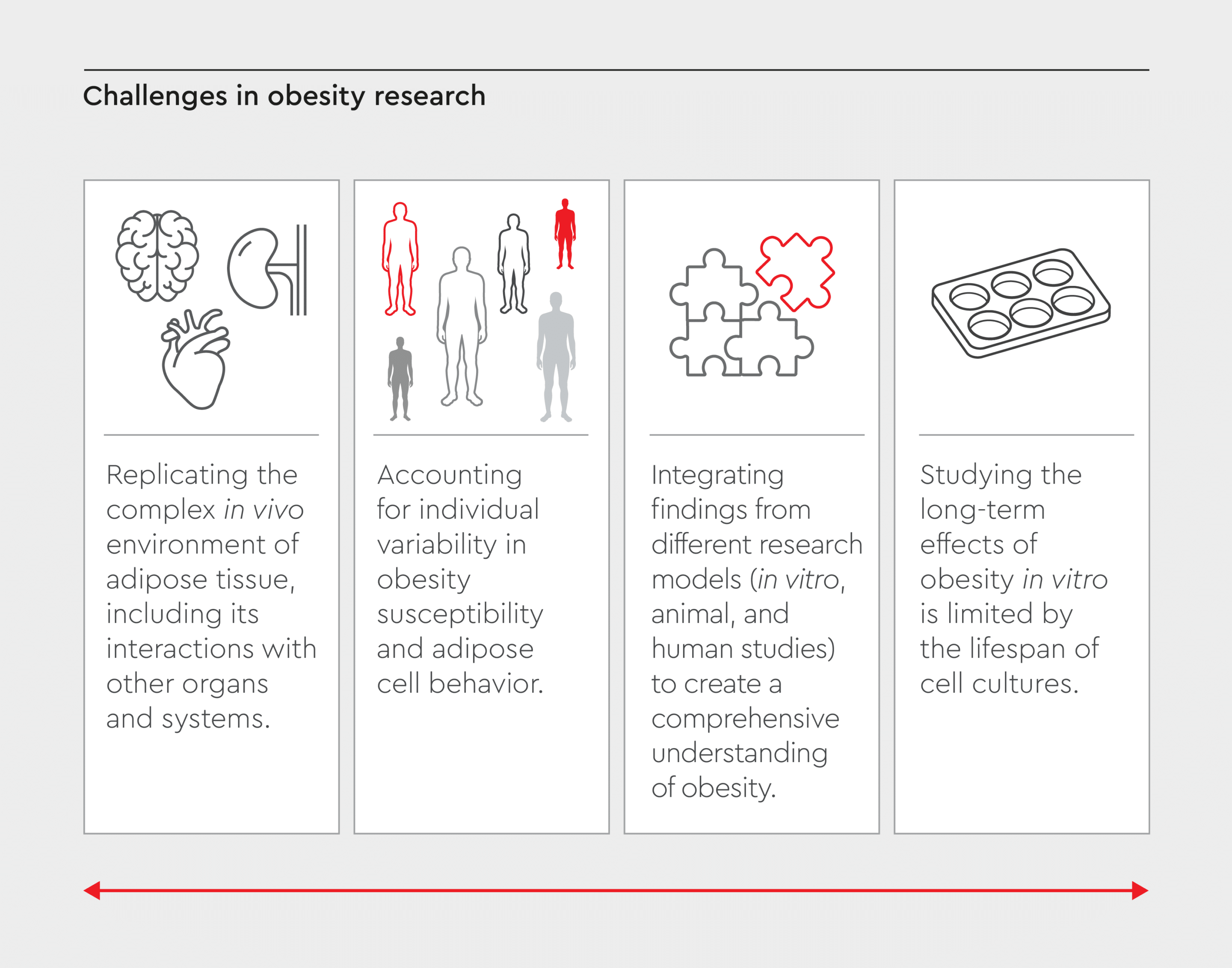
Figure 3: Challenges in obesity research.
Obesity research has advanced in understanding adiposity and its comorbidities, but challenges remain in developing more effective treatments and interventions.
Future perspectives in obesity research
Obesity research is advancing fast, with several innovative technologies and new directions:- Integration of artificial intelligence and machine learning in data analysis.27
- Development of “organs-on-a-chip” systems to study adiposity’s systemic effects.28
- Exploration of the gut microbiome’s role in obesity using in vitro models.29
- In-depth investigation of epigenetic changes in adipocytes using advanced sequencing technologies and in vitro models.30
- Use of 3D and organoid cell cultures to obtain mechanistic insights into obesity and better understand adipose tissue biology.31,32
- Use of primary cells, such as human mesenchymal stem cells isolated from adipose tissue (hMSC-AT), to study obesity-related mechanisms and reduce reliance on cell lines.33
How do we empower your in vitro obesity research?
We offer a range of standardized, high-quality reagents and cells specifically designed for in vitro obesity research (Figure 4):- Human White Preadipocytes (HWP): These primary cells are ideal for studying adipocyte differentiation and function.
- Preadipocyte Differentiation Medium: Optimized for efficient differentiation of preadipocytes into mature adipocytes.
- Preadipocyte Growth Medium: Supports the expansion of preadipocytes while maintaining their differentiation potential.
- Adipocyte Nutrition Medium: Specially formulated to maintain mature adipocytes in culture.

Figure 4: Our products for in vitro obesity research.
We offer preadipocytes from subcutaneous or visceral fatty tissue of individual donors, as well as a ready-to-use adipocyte media to ensure optimal cell growth, differentiation and maintenance.
References
Expand
1. Mohajan D, Mohajan HK. Obesity and its related diseases: a new escalating alarming in global health. J Innov Med Res. 2023;2(3):12-23. 2. Malavazos AE, Scravaglieri V, Boniardi F, Meregalli C, Dubini C. Obesity, a disease that deserves clinical awareness. Eur J Prev Cardiol. 2024;31(10):1274-1276. doi:10.1093/eurjpc/zwae205 3. Pati S, Irfan W, Jameel A, Ahmed S, Shahid RK. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers (Basel). 2023;15(2). doi:10.3390/cancers15020485 4. Jorm AF, Korten AE, Christensen H, Jacomb PA, Rodgers B, Parslow RA. Association of obesity with anxiety, depression and emotional well‐being: a community survey. Aust N Z J Public Health. 2003;27(4):434-440. 5. Lee JE, Nam CM, Lee SG, Park S, Kim TH, Park E-C. The economic burden of cancer attributable to obesity in Korea: A population-based cohort study. Eur J Cancer Care (Engl). 2019;28(5):e13084. doi:10.1111/ecc.13084 6. Speakman JR. Obesity: the integrated roles of environment and genetics. J Nutr. 2004;134(8):2090S-2105S. 7. Ruiz-Ojeda FJ, Rupérez AI, Gomez-Llorente C, Gil A, Aguilera CM. Cell models and their application for studying adipogenic differentiation in relation to obesity: a review. Int J Mol Sci. 2016;17(7):1040. 8. Guilherme A, Henriques F, Bedard AH, Czech MP. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat Rev Endocrinol. 2019;15(4):207-225. doi:10.1038/s41574-019-0165-y 9. Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23(2):120-133. doi:10.1038/s41576-021-00414-z 10. Pamplona JH, Zoehler B, Shigunov P, et al. Alternative methods as tools for obesity research: In vitro and in silico approaches. Life. 2022;13(1):108. 11. Saeidnia S, Manayi A, Abdollahi M. From in vitro experiments to in vivo and clinical studies; pros and cons. Curr Drug Discov Technol. 2015;12(4):218-224. 12. Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu Rev Nutr. 2011;31(1):33-47. 13. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129-139. 14. Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance–a mini-review. Gerontology. 2009;55(4):379-386. 15. Lefterova MI, Zhang Y, Steger DJ, et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22(21):2941-2952. 16. Czech MP. Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol Metab. 2020;34:27-42. 17. Shen JX, Couchet M, Dufau J, et al. 3D adipose tissue culture links the organotypic microenvironment to improved adipogenesis. Adv Sci. 2021;8(16):2100106. 18. Lauschke VM, Hagberg CE. Next-generation human adipose tissue culture methods. Curr Opin Genet Dev. 2023;80:102057. 19. Hu W, Jiang C, Guan D, et al. Patient adipose stem cell-derived adipocytes reveal genetic variation that predicts antidiabetic drug response. Cell Stem Cell. 2019;24(2):299-308. 20. Huang Z, Xu A. Adipose extracellular vesicles in intercellular and inter-organ crosstalk in metabolic health and diseases. Front Immunol. 2021;12:608680. 21. Kwan HY, Chen M, Xu K, Chen B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell Mol Life Sci. 2021:1-14. 22. Matilainen J, Berg V, Vaittinen M, et al. Increased secretion of adipocyte-derived extracellular vesicles is associated with adipose tissue inflammation and the mobilization of excess lipid in human obesity. J Transl Med. 2024;22(1):623. 23. Kumar V, Kiran S, Kumar S, Singh UP. Extracellular vesicles in obesity and its associated inflammation. Int Rev Immunol. 2022;41(1):30-44. doi:10.1080/08830185.2021.1964497 24. Tanzi MC, Farè S. Adipose tissue engineering: state of the art, recent advances and innovative approaches. Expert Rev Med Devices. 2009;6(5):533-551. 25. Novakofski J. Adipogenesis: usefulness of in vitro and in vivo experimental models. J Anim Sci. 2004;82(3):905-915. 26. Dufau J, Shen JX, Couchet M, et al. In vitro and ex vivo models of adipocytes. Am J Physiol Physiol. 2021. 27. An R, Shen J, Xiao Y. Applications of artificial intelligence to obesity research: scoping review of methodologies. J Med Internet Res. 2022;24(12):e40589. 28. Jang M, Kim HN. From single-to multi-organ-on-a-chip system for studying metabolic diseases. BioChip J. 2023;17(2):133-146. 29. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121(6):2126-2132. 30. Barilla S, Treuter E, Venteclef N. Transcriptional and epigenetic control of adipocyte remodeling during obesity. Obesity. 2021;29(12):2013-2025. 31. Taylor J, Sellin J, Kuerschner L, et al. Generation of immune cell containing adipose organoids for in vitro analysis of immune metabolism. Sci Rep. 2020;10(1):21104. doi:10.1038/s41598-020-78015-9 32. Baganha F, Schipper R, Hagberg CE. Towards better models for studying human adipocytes in vitro. Adipocyte. 2022;11(1):413-419. doi:10.1080/21623945.2022.2104514 33. Herbers E, Patrikoski M, Wagner A, et al. Preventing white adipocyte browning during differentiation in vitro: the effect of differentiation protocols on metabolic and mitochondrial phenotypes. Stem Cells Int. 2022;2022(1):3308194. doi:https://doi.org/10.1155/2022/3308194
Contact our experts Are you a researcher or clinical scientist working on obesity? Contact our specialists to learn how our products can accelerate your in vitro obesity research.
