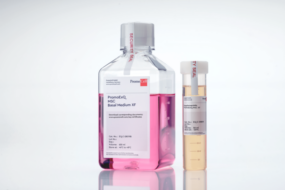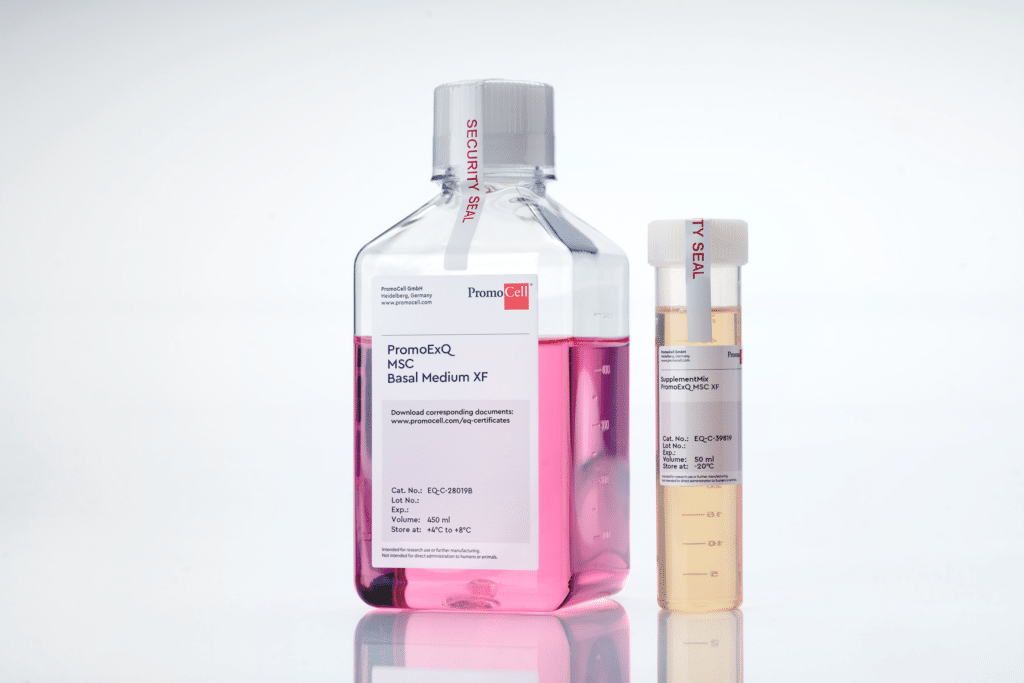
Our new PromoExQ MSC Growth Medium XF is a serum- and xeno-free medium suitable for your cell therapy manufacturing applications.

It is manufactured under the highest quality standards in compliance with our EXCiPACTTM GMP certification scheme, which builds on our existing ISO 9001:2015 quality management system.
Our medium was optimized for the in vitro expansion of human mesenchymal stem cells MSCs in GMP regulated environment, providing consistent growth and maintenance of a variety of multipotent MSCs, including MSCs from bone marrow, umbilical cord matrix (Wharton’s jelly), and adipose tissue.
Our PromoExQ MSC Growth Medium XF comes with relevant supporting documentation free of charge. There is no need to chase after the documents through several emails for your risk assessment process.
The documentation set includes:
- Excipient Quality Assessment File (EQAF)
- Statement of GMP compliance
- Statement on microbial and viral safety (MVS)
- Statement on BSE/TSE status
- Certificate of origin (CoO)
- Declaration of conformity
- Statement of genetically modified organisms (GMO)
- Certificate of analysis (CoA)
Go to our product page and place your order now:
To enable customization and upscaling of PromoExQ MSC Growth Medium XF, we offer:
- Customized media formulation
- Additional quality tests
- Production of large media batches in bigger bottle formats or media bags
- Bulk production of media supplements
Do you need an individually tailored solution?
“We are proud to offer our customers a product that is essential for the success of their cell therapy manufacturing applications.
With our new PromoExQ MSC Growth Medium XF, it was our highest priority to provide all required documents quickly and easily, together with the GMP grade medium. We know from our customers how critical it is for the regulatory process to perform a risk assessment at the earliest stage of their studies. By providing the required documentation, we help our clients to save time and money. With our expertise and the new product, we can advance research and development in this field and contribute to improving healthcare.”
– Managing Director Dr. Irma Börcsök
We ensure consistent lot-to-lot performance and proactively inform you about changes in raw material specifications, which may impact the regulatory status. With each medium batch, you also receive complete documentation, including a Certificate of Origin.
We further facilitate the transition to a GMP-compliant process by offering a standard version of our PromoExQ MSC Growth Medium XF. The release specifications of the functional performance assays of the research grade medium are identical to the GMP grade cell culture medium, for an easy switch from the standard product to the GMP grade version.