Mesenchymal stem cells (MSCs)
Mesenchymal stem cells (MSCs) are multipotent adult stem cells known for their ability to self-renew and differentiate into various cell types.1–4 These cells have gained significant attention in regenerative medicine due to their unique properties and potential applications in stem cell-based treatments and tissue repair.3,4

What is a mesenchymal stem cell?
MSCs are non-hematopoietic stem cells that were initially isolated from human bone marrow.1 They can differentiate into tissues of both mesenchymal and non-mesenchymal origin, including osteoblasts, chondrocytes, adipocytes, and even neuronal and cardiomyogenic lineages.1–4 Their differentiation potential makes them attractive for use in cell-based therapies.4
Stem cells vs. stromal cells
The term "mesenchymal stem cells" was originally proposed by Caplan in 1991 due to their multilineage differentiation potential.3 However, Caplan later suggested using the term “mesenchymal stromal cells” to better reflect their biological characteristics and functions.5 Despite this, the terms “stem” and “stromal” are often used interchangeably when referring to MSCs, and both are widely accepted in scientific literature and practice.6,7
For consistency, we use the term “stem” throughout our website and products. The International Society for Cellular Therapy (ISCT) is working to standardize the terminology.
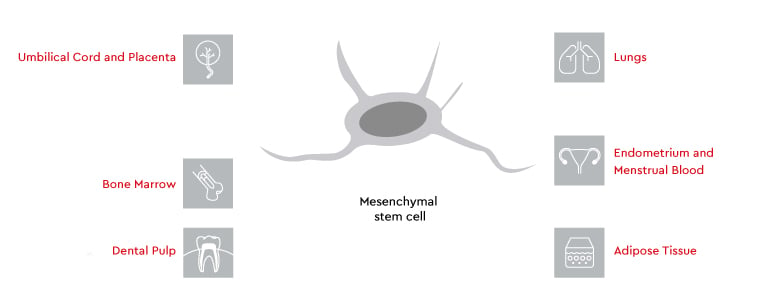
Sources of mesenchymal stem cells
Where do mesenchymal stem cells come from? MSCs can be found in various tissues in the human body. While bone marrow was the first identified source of MSCs, researchers have discovered that MSCs can also be found in several other tissues, each offering unique properties.
Sources of MSCs include8–12:
- Bone marrow (most common)
- Adipose tissue
- Umbilical cord blood
- Umbilical cord tissue
- Placenta
- Dental pulp
- Synovial fluid
- Peripheral blood

Wharton’s jelly from the umbilical cord is considered a rich source of young and primitive MSCs.11 The diverse sources of MSCs make them a flexible tool for scientific research and therapeutic applications.
Biological properties of MSCs
Mesenchymal stem cells exhibit remarkable properties that make them a key focus in regenerative medicine. Their ability to differentiate into various cell types, modulate immune responses, and secrete bioactive molecules has positioned them as the cornerstone of cell-based therapies.
Mechanisms of action
MSCs exert their therapeutic effects through multiple mechanisms1–7:
- Differentiation into various cell types
- Secretion of bioactive molecules (paracrine effects)
- Immunomodulation to suppress immune responses
- Homing to sites of injury or inflammation
How do mesenchymal stem cells from different tissues differ in terms of their biological function?
Although human MSCs derived from the bone marrow, adipose tissue, and umbilical cord matrix are from different sources, they have similar biological properties and functions. Depending on the tissue of origin, they may have a higher preference for differentiation into one particular cell type and a lower preference for another. However, they all still retain the differentiation potential for the mesenchymal lineage.13
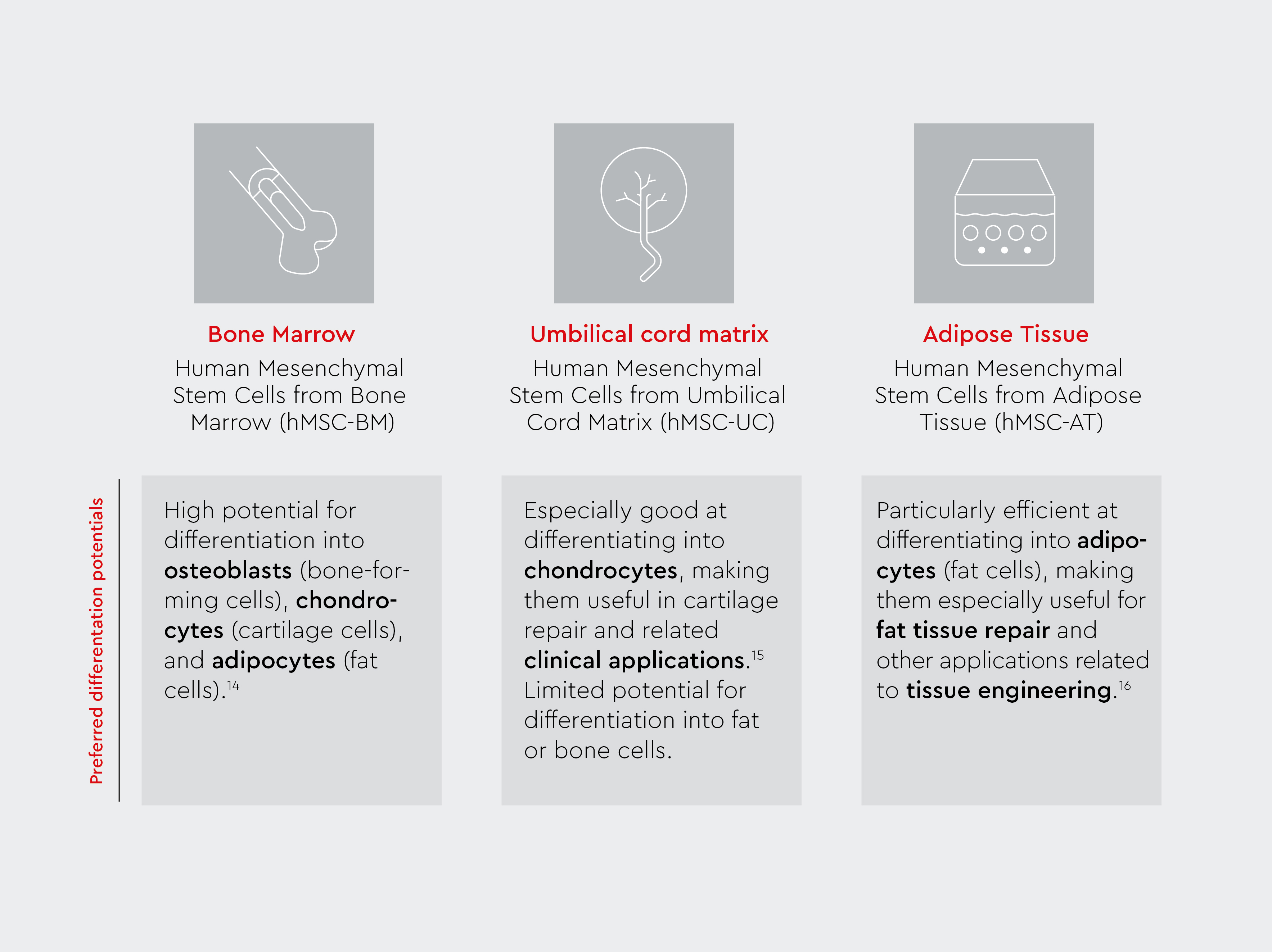

MSC-UC are often considered more primitive and youthful than MSCs derived from bone marrow or adipose tissue, contributing to greater differentiation potential and improved regenerative capabilities.13–16 Unlike embryonic stem cells, MSC-UC are obtained from umbilical cord tissues, which raises fewer ethical concerns as they are collected after childbirth in a non-invasive manner.8
Isolation and culturing techniques
MSCs can be isolated using various methods, including11:
- Bone marrow aspiration
- Liposuction (for adipose-derived MSCs)
- Collection of umbilical cord tissue at birth
- Apheresis (for peripheral blood MSCs)
Once isolated, they are cultured under specific conditions to maintain their stem cell properties and expand their numbers for therapeutic use.
Our MSC portfolio includes hMSC and media to support growth and differentiation of MSCs. In this brochure, you’ll find all the tools – cells, media, and guidance – you need for mesenchymal stem cell culture work.
Characterization and differentiation potential
According to the International Society for Cellular Therapy (ISCT), MSCs are characterized by:8–11
- Plastic adherence in culture
- Expression of specific surface markers (CD105, CD73, CD90)
- Lack of expression of certain markers (CD45, CD34, CD14, CD11b, CD79α, CD19, HLA-DR)
- Ability to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro
Want to learn more about the specific markers and methods used to identify and assess the quality of mesenchymal stem cells?
Read the blog article:
Immunomodulatory properties
MSCs possess potent immunomodulatory capabilities, allowing them to:11
- Suppress immune responses
- Reduce inflammation
- Promote tissue repair and regeneration
These properties make MSCs valuable for the treatment of autoimmune diseases and inflammatory conditions.
Interested in learning more about how mesenchymal stem cells interact with the immune system to promote healing and reduce inflammation?
Watch the video:
Applications of MSCs
Mesenchymal stem cells have shown promise in treating various conditions, including1–4:
- Graft-versus-host disease (GvHD)
- Myocardial infarction
- Cartilage and bone repair
- Skin wound healing
- Neuronal regeneration
- Osteogenesis imperfecta
Ongoing clinical studies are exploring MSC-based therapies for conditions such as osteoarthritis, cardiovascular diseases, and neurological disorders, advancing cell-based therapies and stem cell research.1–4
Stay informed about MSC research
Are you working with Mesenchymal Stem Cells (MSCs) – or planning to? Let us know which aspects of MSC cell culture you’re currently focused on.
FAQs regarding mesenchymal stem cells
1. Are MSCs actually stem cells?
While debated, MSCs exhibit key stem cell characteristics such as self-renewal capacity and multipotency.72. How are they different from embryonic stem cells?
Mesenchymal stem cells are adult stem cells, whereas embryonic stem cells are derived from blastocysts. MSCs are multipotent and, therefore, have more limited differentiation potential than pluripotent embryonic stem cells, but they present fewer ethical concerns.83. What are the benefits of MSCs?
MSCs offer potential for tissue repair, immunomodulation, and treatment of various diseases, including those requiring stem cell transplantation.1–44. What are the disadvantages of MSCs?
Disadvantages of MSCs include potential for uncontrolled differentiation, limited engraftment, and possible tumor formation in some cases.75. What diseases can MSCs treat?
Diseases that could be treated using MSC-based therapies include GvHD, bone and cartilage disorders, cardiovascular diseases, and some autoimmune conditions.1,2,46. How do MSCs work?
MSCs can promote regeneration and tissue repair through cell differentiation, paracrine effects, immunomodulation, and homing to injured sites.1–77. What are the concerns regarding MSC therapy?
Concerns include potential for tumor formation, limited engraftment, and variability in cell quality.78. How can MSC therapy be standardized and regulated?
There are ongoing efforts to establish uniform protocols for isolation, expansion, and characterization of MSCs for clinical use.7 In addition, ISCT has put forth characterization criteria to facilitate standardized MSC isolation and ensure their identity.9. What is the distinction between pericytes and MSCs?
Pericytes are classified as ‘mesenchymal-like cells’. They are very similar to MSCs but do not have the same properties. For example, in contrast to MSCs, pericytes are involved in angiogenesis.17–19
References
Expand
- Rastegar, F., Shenaq, D., Huang, J., Zhang, W., Zhang, B. Q., He, B. C., Chen, L., Zuo, G. W., Luo, Q., Shi, Q., Wagner, E. R., Huang, E., Gao, Y., Gao, J. L., Kim, S. H., Zhou, J. Z., Bi, Y., Su, Y., Zhu, G., Luo, J., … He, T. C. (2010). Mesenchymal stem cells: Molecular characteristics and clinical applications. World journal of stem cells, 2(4), 67–80. https://doi.org/10.4252/wjsc.v2.i4.67
- Berebichez-Fridman, R., & Montero-Olvera, P. R. (2018). Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos University medical journal, 18(3), e264–e277. https://doi.org/10.18295/squmj.2018.18.03.002
- Najar, M., Melki, R., Khalife, F., Lagneaux, L., Bouhtit, F., Moussa Agha, D., Fahmi, H., Lewalle, P., Fayyad-Kazan, M., & Merimi, M. (2022). Therapeutic Mesenchymal Stem/Stromal Cells: Value, Challenges and Optimization. Frontiers in cell and developmental biology, 9, 716853. https://doi.org/10.3389/fcell.2021.716853
- Bobis, S., Jarocha, D., & Majka, M. (2006). Mesenchymal stem cells: characteristics and clinical applications. Folia histochemica et cytobiologica, 44(4), 215–230.
- Caplan A. I. (2017). Mesenchymal Stem Cells: Time to Change the Name!. Stem cells translational medicine, 6(6), 1445–1451. https://doi.org/10.1002/sctm.17-0051
- Costela-Ruiz, V. J., Melguizo-Rodríguez, L., Bellotti, C., Illescas-Montes, R., Stanco, D., Arciola, C. R., & Lucarelli, E. (2022). Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. International Journal of Molecular Sciences, 23(11), 6356. https://doi.org/10.3390/ijms23116356
- Naji, A., Eitoku, M., Favier, B., Deschaseaux, F., Rouas-Freiss, N., & Suganuma, N. (2019). Biological functions of mesenchymal stem cells and clinical implications. Cellular and molecular life sciences: CMLS, 76(17), 3323–3348. https://doi.org/10.1007/s00018-019-03125-1
- Gopalarethinam, J., Nair, A. P., Iyer, M., Vellingiri, B., & Subramaniam, M. D. (2023). Advantages of mesenchymal stem cell over the other stem cells. Acta histochemica, 125(4), 152041. https://doi.org/10.1016/j.acthis.2023.152041
- Hass, R., Kasper, C., Böhm, S., & Jacobs, R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell communication and signaling: CCS, 9, 12. https://doi.org/10.1186/1478-811X-9-12
- Wruck, W., Graffmann, N., Spitzhorn, L. S., & Adjaye, J. (2021). Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Acquire Rejuvenation and Reduced Heterogeneity. Frontiers in cell and developmental biology, 9, 717772. https://doi.org/10.3389/fcell.2021.717772
- Frausin, S., Viventi, S., Verga Falzacappa, L., Quattromani, M. J., Leanza, G., Tommasini, A., & Valencic, E. (2015). Wharton's jelly derived mesenchymal stromal cells: Biological properties, induction of neuronal phenotype and current applications in neurodegeneration research. Acta histochemica, 117(4-5), 329–338. https://doi.org/10.1016/j.acthis.2015.02.005
- Pittenger, M. F., Discher, D. E., Péault, B. M., Phinney, D. G., Hare, J. M., & Caplan, A. I. (2019). Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative medicine, 4, 22. https://doi.org/10.1038/s41536-019-0083-6
- Wagner, W., Wein, F., Seckinger, A., Frankhauser, M., Wirkner, U., Krause, U., Blake, J., Schwager, C., Eckstein, V., Ansorge, W., & Ho, A. D. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental hematology, 33(11), 1402–1416. https://doi.org/10.1016/j.exphem.2005.07.003
- Al-Nbaheen, M., Vishnubalaji, R., Ali, D., Bouslimi, A., Al-Jassir, F., Megges, M., Prigione, A., Adjaye, J., Kassem, M., & Aldahmash, A. (2013). Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem cell reviews and reports, 9(1), 32–43. https://doi.org/10.1007/s12015-012-9365-8
- Ma, J., Wu, J., Han, L., Jiang, X., Yan, L., Hao, J., & Wang, H. (2019). Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum-free condition. Stem cell research & therapy, 10(1), 19. https://doi.org/10.1186/s13287-018-1104-x
- Monaco, E., Bionaz, M., Hollister, S. J., & Wheeler, M. B. (2011). Strategies for regeneration of the bone using porcine adult adipose-derived mesenchymal stem cells. Theriogenology, 75(8), 1381–1399. https://doi.org/10.1016/j.theriogenology.2010.11.020
- Cantoni, S., Bianchi, F., Galletti, M., Olivi, E., Alviano, F., Galiè, N., & Ventura, C. (2015). Occurring of In Vitro Functional Vasculogenic Pericytes from Human Circulating Early Endothelial Precursor Cell Culture. Stem cells international, 2015, 943671. https://doi.org/10.1155/2015/943671
- Kobolak, J., Dinnyes, A., Memic, A., Khademhosseini, A., & Mobasheri, A. (2016). Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods (San Diego, Calif.), 99, 62–68. https://doi.org/10.1016/j.ymeth.2015.09.016
- Blocki, A., Beyer, S., Jung, F., & Raghunath, M. (2018). The controversial origin of pericytes during angiogenesis - Implications for cell-based therapeutic angiogenesis and cell-based therapies. Clinical hemorheology and microcirculation, 69(1-2), 215–232. https://doi.org/10.3233/CH-189132
