
3D Cell Culture
Find suitable products, scientific resources or answers to your questions within our technical library FAQ.
Checkout using your account
This form is protected by reCAPTCHA - the Google Privacy Policy and Terms of Service apply.
Checkout as a new customer
Creating an account has many benefits:
Delivering physiologically relevant research data relies on models as close to the target biological system as possible. 3D cellular models allow a better understanding of complex biology in a physiologically relevant context. At PromoCell, we have both the expertise and the appropriate systems to support your journey towards the future of in vitro research.
Working with 3D cell culture better mimics the in vivo environment than classical 2D culture (Cukierman E., et al. 2001). Biological processes typically involve multiple cell types and due to their architectural similarity to in vivo tissues, 3D culture can recreate some of this complexity in vitro (Pampaloni F., et al. 2007).
3D cell culture refers to the culture of living cells in three dimensions, mimicking tissue and organ-specific microarchitecture (Huh D., et al. 2011). 3D cell growth allows better intercellular contact and signaling (Abbott A. 2003) while allowing cells to differentiate into more complex structures (Cukierman E., et al. 2002).
There are various methods for 3D cell culture, each with its own advantages and limitations. Since the choice of a method appropriate for a specific cell-based assay is key for success, in this article we will review four different approaches to 3D cell culture, namely, spheroids, organoids, air-liquid interface, and magnetic 3D culture.
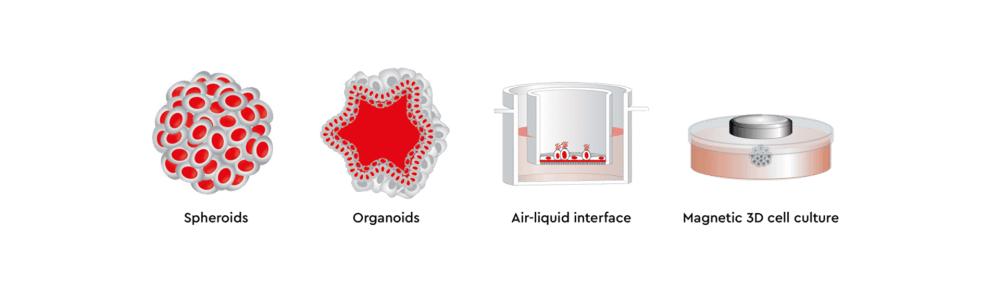

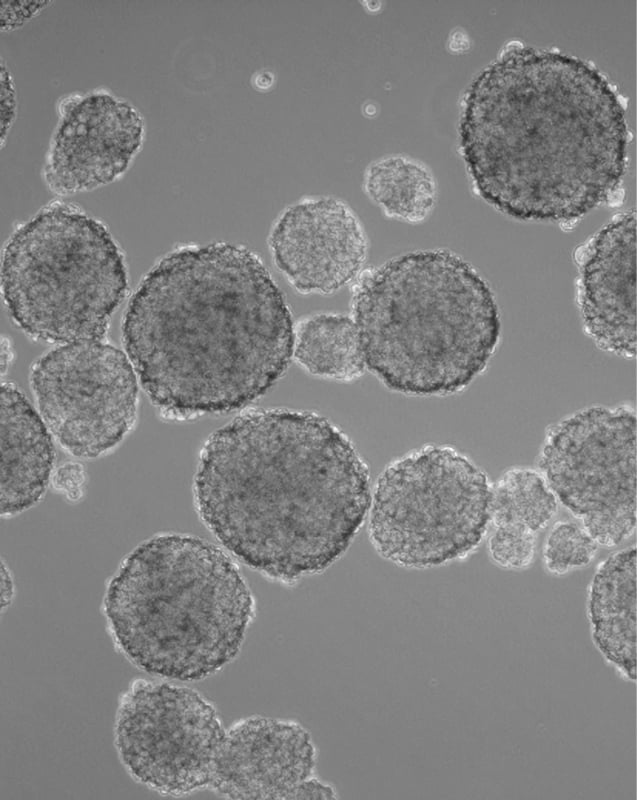

A spheroid culture system facilitates cell-cell and cell-matrix interactions to overcome the limitations of traditional monolayer cell cultures. In suspension, aggregates of adjacent cells form a spheroid shape useful in tumor and cancer research, therapeutic transplantation, drug screening, and clinical research (Ryu N.E., et al. 2019). In fact, spheroids are the most commonly used 3D model for cancer research as they are reproducible and scalable, which can accelerate drug development and drug screening times in oncology.
Spheroids can be developed from commercially available cancer cell lines or primary cancer cells from tumor biopsies. Growing cancer cells over several passages in 3D culture, as so-called tumorspheres, leads to an enrichment of cancer stem cells, a small subpopulation within a tumor that drives tumor progression and metastasis. Due to their inherent biological features, such as anoikic resistance and self-renewal, cancer stem cells are able to form tumorspheres from single-cell cultures. Continuous proliferation is also supported during serial passage of tumorsphere cultures, making this type of 3D cancer cell culture applicable for in vitro models of metastasis.
WHAT WE OFFER
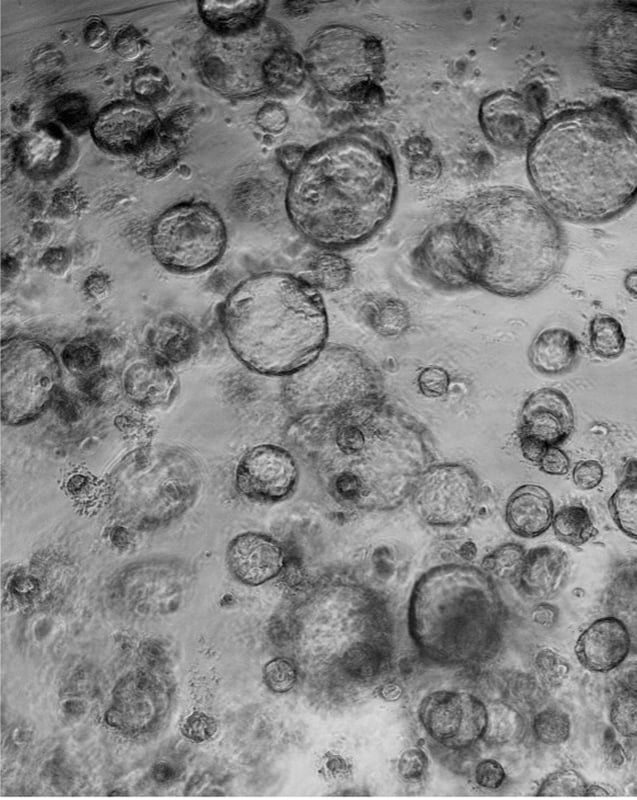
Organoids are in vitro 3D cell aggregates derived from primary tissue or stem cells that provide reproducible and scalable 3D models. They can self-organize three-dimensionally owing to their self-renewal and differentiation capacities in a particular 3D microenvironment in response to physical and chemical cues (Lancaster M. A. and J. A. Knoblich 2014), such as an extracellular matrix hydrogel (basement membrane extract or collagen).
Several organ architectures have been copied using this technique, including cerebral, intestinal, lung, or kidney tissue (Lancaster M. A., et al. 2013; Sato T., et al. 2009; Lee J. H., et al. 2014; Takasato M., et al. 2015). For instance, it is possible to develop a 3D cancer organoid model using various cell types like fibroblasts and mammary carcinoma cells. Or even functional airway organoids with apical-out or inward-oriented cilia formation based on primary human bronchial epithelial cells, as can be observed in the following videos.
WHAT WE OFFER
Functional 3D human airway organoids from primary human bronchial epithelial cells (HBEpC) using our Air-Liquid Interface Medium (ALI-Airway):
Reproducible and scalable 3D cancer organoids in our Cancer Cell Line Medium XF:

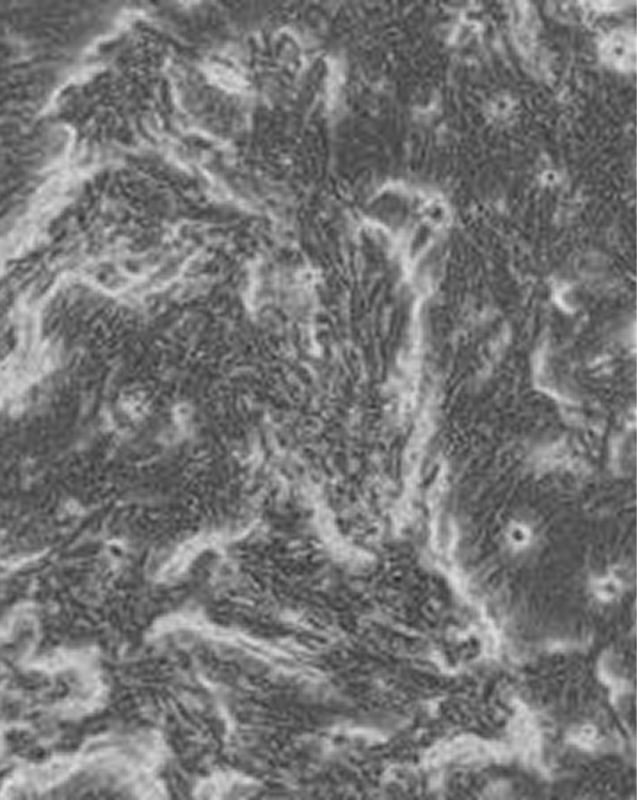

An air-liquid interface (ALI) is advantageous for studying the physiological and pathophysiological responses of the respiratory tract, specifically how cells respond to aerosolized drugs. Particularly, growing primary cells at an ALI is a good approach as the transcriptional profile of differentiated primary cells grown at the ALI very closely resemble the in vivo airway epithelium. In addition, the similarity between cells of tracheal and bronchial origin within and between human donors suggests a robust expression profile specific to airway cells (Pezzulo, A.A., et al. 2010).
Another advantage of ALI culture is that it allows for the polarization of epithelial cells by supporting their differentiation into a pseudostratified epithelium that shows a barrier function between adjacent environments. Measuring the integrity of this barrier function can be used as an indicator of cell monolayer integrity before it is used to study drug uptake or cytotoxicity.
WHAT WE OFFER
A standardized Air-Liquid Interface Culture System, consisting of our Air-Liquid Interface Medium (ALI-Airway) and ALI pre-screened HBEpCs
Magnetic 3D cell cultures are high-throughput screening cell models produced by a bioprinting method that uses biocompatible magnetic nanoparticles to print cells into 3D structures. In magnetic 3D cell culture, cells are tagged with magnetic nanoparticles. Once magnetic, these cells can be rapidly printed into specific 3D patterns using external magnetic forces to mimic tissue structure and function.
This technique is advantageous not only because of its speed and fine spatial control but also because the extracellular matrix results from endogenous synthesis, not requiring artificial substrates (scaffold-free) (Souza G.R., et al. 2010). In addition, this technique is not restricted to specific cell types, which makes it broadly applicable.
An area of research which greatly benefits from this 3D cell culture technique is skin modeling (dermatology), particularly for wound healing. 2D in vitro models cannot mimic the native cellular environment and do not consider the impact of drug testing on the various phases of the healing process. To address this issue, a collaboration between PromoCell, CytoSMART, and Grenier BioOne led to the development of a new high-throughput 3D wound healing model that uses co-cultured skin cells. In this model, changing ratios of keratinocytes and fibroblasts mimics the different phases of the wound healing process.
WHAT WE OFFER
Detailed instructions for the generation of a high-throughput 3D wound healing model using


Have a look at other Research Areas
PromoCell uses HubSpot to provide LiveChat support. This feature is currently blocked due to your cookie preferences.
Accept cookies to enable LiveChat