Throughout 2025, our products were featured in a wide range of research areas, such as oncology, immunology, and cardiovascular research. This blog post highlights a selection of recent scientific papers utilizing our products. It illustrates how researchers apply physiological relevant cell models and defined culture systems to investigate disease mechanisms, therapeutic strategies, and human biological function. These references provide a year-end snapshot of how scientists integrate our products into experimental workflows spanning basic research through translational models.
Research highlights across biological domains
By supporting research across multiple domains, PromoCell helps accelerate breakthroughs that deepen our understanding of health and disease.
Targeting cancer stem cells to improve lung cancer therapy
Cancer stem cells (CSCs) play a pivotal role in the initiation and progression of malignant tumors and contribute to the development of chemoresistance and metastasis. CSCs promote tumor growth and resistance to conventional therapies by regulating the tumor microenvironment. A recent study from the University of Pittsburgh showed that triple therapy delivered using a nanocarrier could reverse CSC-associated chemoresistance in lung cancer.1 The study findings suggest that a nanocomplex consisting of PASA (an anti-inflammatory prodrug carrier based on the COX-2 inhibitor 5-ASA), cisplatin, and MK-2206 (an AKT1 inhibitor) is a promising tool for eliminating the most proliferative tumor cells and counteracting inflammation by inhibiting the functions of CSCs.
We are happy to see our 3D Tumoresphere Medium XF used in this critical effort to target the protumorigenic properties of CSCs and their ability to modulate the tumor microenvironment. Understanding the mechanisms underlying CSC-driven chemoresistance is crucial for targeting the ability of CSCs to promote cancer progression.


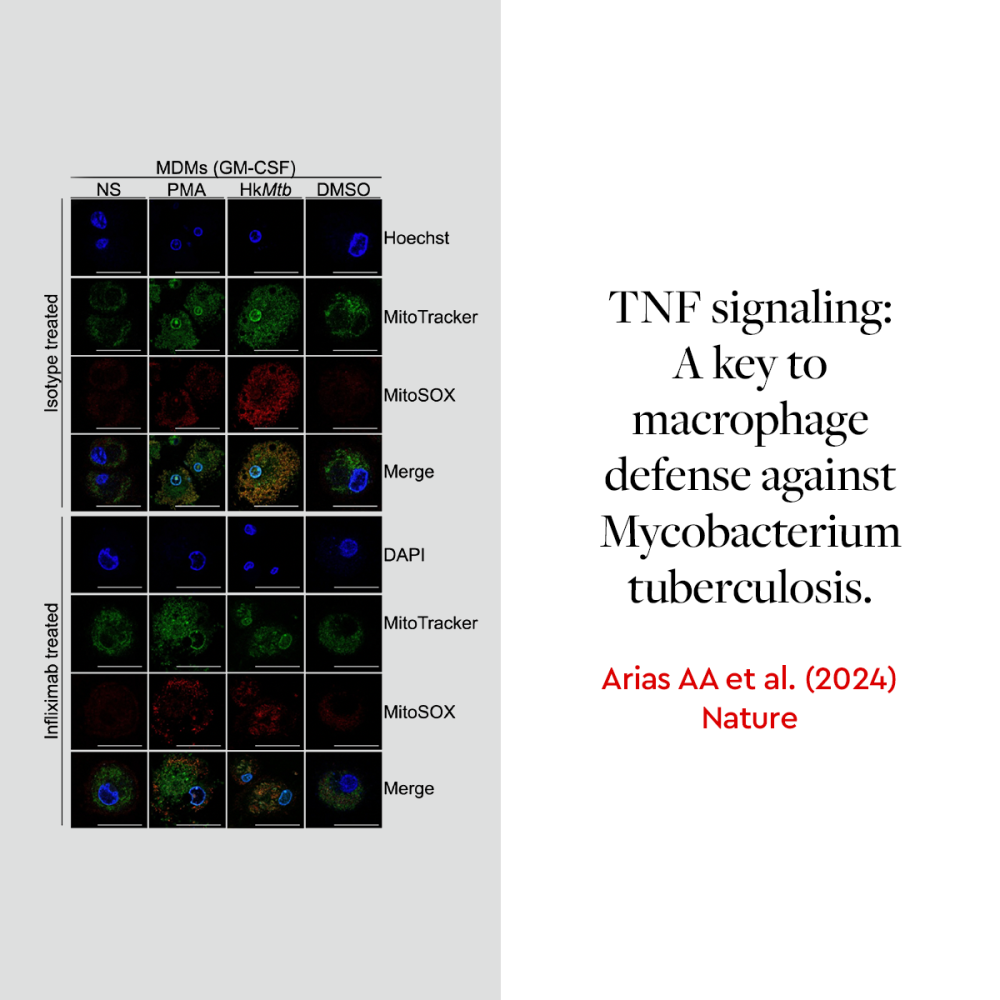
TNF–TNFR1 is essential for defense against tuberculosis
Macrophages act as the body’s first line of defense against infections. Their ability to produce reactive oxygen species (ROS) is critical for fighting pathogens like Mycobacterium tuberculosis. A recent study published in Nature highlights the role of TNF signaling in macrophage function, particularly in ROS production in individuals with tuberculosis.2 Using our Macrophage Generation Medium XF to generate GM-CSF-matured macrophages, researchers from Rockefeller University and the University of Antioquia UdeA demonstrated that TNF-deficient macrophages exhibit impaired ROS production, which compromises their ability to fight Mycobacterium tuberculosis. The study also revealed that TNF signaling through TNFR1 is essential for activating the NADPH oxidase complex, a key mechanism in macrophage-mediated pathogen defense.
We are proud that our Macrophage Generation Medium XF supported this research, helping to advance our understanding of macrophages and their role in infectious diseases.

Advancing multiple myeloma research with 3D models
Multiple myeloma progression is strongly influenced by interactions between tumor cells and their bone marrow microenvironment. These complex relationships with both cellular and non-cellular components are key factors in tumor progression and drug resistance development. In a recent study, researchers from the Valencia Polytechnic University successfully developed a 3D microenvironment model that captures the main components of the multiple myeloma tumor microenvironment.3 Using our bone marrow mesenchymal stem cells (MSCs) and MSC Growth Medium 2, researchers created a 3D platform where multiple myeloma cells interact with both MSCs and extracellular matrix components.
The results demonstrated that this model maintains high cell viability while enabling both direct and indirect interactions between tumor cells and their microenvironment, closely mimicking in vivo conditions.
This approach provides a valuable tool for more representative drug screening assays and personalized treatment strategies for patients with multiple myeloma.


T cell therapy shows promise against solid tumors
Despite advances in cancer immunotherapy, solid tumors often develop resistance to the treatment. A new study published in Nature Medicine demonstrates how engineered T cells can effectively target multiple solid tumor types, including melanoma, sarcoma, and ovarian cancer.4 Researchers from Immatics developed IMA203, an autologous T cell therapy targeting PRAME, a cancer-associated antigen expressed across various solid tumors. The phase 1 trial showed a 52.5% overall response rate across multiple cancer types and 70% in patients with melanoma. Our primary cells from healthy tissues played an important role in this study, enabling researchers to test T cell functionality through co-culture experiments and IFN-γ release ELISA assays, helping ensure the therapy’s safety and specificity.
We’re proud that our products contributed to this promising advancement in cancer immunotherapy that could potentially expand treatment options for patients with difficult-to-treat solid tumors.

Reversing cardiac fibrosis through modulation of mechanosensing pathways
Can fibrosis be reversed? A new study published in Nature suggests that inhibition of stromal mechanosensing can suppress cardiac fibrosis.5 Cardiac fibrosis, characterized by the thickening and stiffening of heart tissue due to excessive production of extracellular matrix, is a key feature of many heart diseases. Once activated, cardiac fibroblasts initiate this pathological process by transforming into myofibroblasts, which contributes to reduced cardiac function and ultimately leads to heart failure.
But what if fibroblast activation could be reversed?
A study from Stanford University challenges a long-standing belief in heart disease research.5 The scientists showed that it’s possible to reverse fibrosis by combining two treatments: one that blocks mechanical sensing through SRC, and another that inhibits TGFβ signaling. Together, these interventions reprogrammed fibroblasts into a resting, non-fibrotic state. In both lab-grown cardiac tissues and mouse models, the approach reduced fibrosis and improved heart function. To support their in vitro work, the researchers used our Fibroblast Growth Medium 3 (FGM3) to culture primary human cardiac fibroblasts. FGM3 was used in multiple critical experiments, including siRNA-mediated knockdowns, live-cell imaging of MRTFA localization, and matrix contraction assays that measured mechanical remodeling.

The findings of this study enhance our understanding of fibroblast plasticity and suggest a therapeutic strategy that extends beyond slowing fibrosis to potentially reversing it. We’re proud that our Fibroblast Growth Medium 3 has contributed to the advancement of heart disease research.
IGF-1 signaling regulates vascular barrier integrity
Insulin-like growth factor-1 (IGF-1) signaling plays an important role in vascular barrier function and atherosclerosis. However, little is known about the mechanisms behind IGF-1’s ability to regulate vascular barrier function.
In a recent study, researchers from the Leeds Institute of Cardiovascular and Metabolic Medicine used mouse models with endothelial-specific overexpression of either wild-type IGF-1 receptors (hIGFREO/ApoE−/−) or signaling-defective mutant receptors (mIGFREO/ApoE−/−), alongside our human umbilical vein endothelial cells (HUVECs) for in vitro analysis.6 The researchers found that when IGF-1 signaling is disrupted, the vascular barrier becomes compromised, allowing cholesterol-rich lipoproteins to penetrate the arterial walls and contribute to the formation of atherosclerotic plaques.
Conversely, enhanced IGF-1 receptor signaling strengthened endothelial junctions and reduced both paracellular leakage and transcellular transport of atherogenic lipoproteins. The study provides mechanistic insights into vascular barrier function and could pave the way for novel therapies targeting endothelial dysfunction in cardiovascular disease.

We are proud that our Cryo-SFM Plus and HUVECs were used in this study exploring endothelial function and atherogenesis. By successfully preserving primary HUVECs and maintaining their cellular and nuclear integrity, Cryo-SFM Plus enabled researchers to create reliable pause points in their experimental workflows, which is crucial for downstream processes such as nuclear extraction used in the study.
How melanoma communicates with blood vessels, and why αvβ5 matters
Melanoma doesn‘t grow in isolation. To expand and metastasize, melanoma cells actively influence their microenvironment, especially the blood vessels that supply nutrients and oxygen. Understanding how tumors trigger vascular growth remains a critical question in cancer biology and therapy development. One mechanism involves ectosomes, extracellular vesicles released by tumor cells that transfer molecular signals into the tumor microenvironment.
In a 2024 study published in Cells, Surman et al. demonstrate that melanoma-derived ectosomes promote angiogenesis predominantly via the αvβ5 integrin–VEGF signaling pathway, rather than the αvβ3 integrin–TNF-α axis.7
This finding refines existing models of tumor-driven vascular activation and highlights integrin specificity in endothelial reprogramming. The study employed physiologically relevant human primary cell models. Normal Human Epidermal Melanocytes served as non-transformed controls for ectosome generation. Endothelial cell behavior was evaluated using HUVEC and HDMEC cultures maintained in our optimized cell culture media. Cell viability, migration, and capillary-like tube formation were assessed using functional assays. By combining ectosome biology, integrin signaling, and human primary cells, the study illustrates how closely physiological model systems can reveal critical aspects of tumor–endothelium communication and vascular pathology.


By combining ectosome biology, integrin signaling, and human primary cells, the study illustrates how closely physiological model systems can reveal critical aspects of tumor–endothelium communication and vascular pathology.
Reliability and continuity in experimental research
Across disease areas and model systems, consistent product performance is foundational to experimental reproducibility. The studies presented here illustrate how specialized media formulations, well-characterized primary cells, and standardized workflows enable researchers to generate interpretable data under controlled conditions. From infectious disease modeling to fibrosis research and cancer biology, the reliability of foundational tools remains central to experimental confidence and method transferability.
Contact our experts
To learn how our products can support your research, explore our full product portfolio or contact our technical support team for application-specific guidance.
References
Expand
- Huang H, Ji B, Huang Y, Li S, Luo Z, Chen S, et al. Advanced hierarchical computational modeling-based rational development of platinum(II) nanocomplex to improve lung cancer therapy. Adv Funct Mater. 2024;2411334
- Arias AA, Neehus AL, Ogishi M, et al. Tuberculosis in otherwise healthy adults with inherited TNF deficiency. Nature. 2024;633(8029):417–425. doi:10.1038/s41586-024-07866-3
- García-Briega MI, Plá-Salom J, Clara-Trujillo S, et al. Co-culture of multiple myeloma cell lines and bone marrow mesenchymal stem cells in a 3D microgel environment. Biomater Adv. 2025;172:214243
- Wermke M, Araujo DM, Chatterjee M, et al. Autologous T cell therapy for PRAME+ advanced solid tumors in HLA-A*02+ patients: a phase 1 trial. Nat Med. 2025. Published online April 9
- Cho S, Rhee S, Madl CM, et al. Selective inhibition of stromal mechanosensing suppresses cardiac fibrosis. Nature. 2025;642:766–775
- Drozd M, Bruns AF, Yuldasheva NY, et al. Endothelial insulin-like growth factor-1 signalling regulates vascular barrier function and atherogenesis. Cardiovasc Res. 2025;121:1108–1120
- Surman M, et al. The proangiogenic effects of melanoma-derived ectosomes are mediated by αvβ5 integrin rather than αvβ3 integrin. Cells. 2024;13(16):1336. doi:10.3390/cells13161336
Related resources
