Traditional cell culture systems often fall short of replicating the complex environment of the human airway, limiting their predictive power in respiratory research. To address these limitations, researchers increasingly rely on physiologically relevant in vitro models that better reflect human airway biology. Among these, air-liquid interface (ALI) culture bridges the gap between simple two-dimensional systems and complex animal models, offering a human-based approach for studying respiratory function and disease in vitro.
What is the air-liquid interface?
The air-liquid interface is the boundary where air meets liquid in biological systems. In the body, this interface occurs naturally at multiple sites, including the lungs, nasal passages, and the eye surface. This boundary zone plays a critical role in maintaining tissue function and barrier integrity.1,2
Compared to fully submerged culture systems, air-liquid interface conditions more accurately replicate the natural environment of airway epithelial cells.1,3 The apical surface is exposed to air, while the basal surface is in contact with tissue fluid and blood vessels.4 This polarity allows for gas exchange, mucus secretion, ciliary motion, and immune surveillance.4 Understanding this architecture in vivo is key to developing in vitro models that accurately represent human airway physiology.
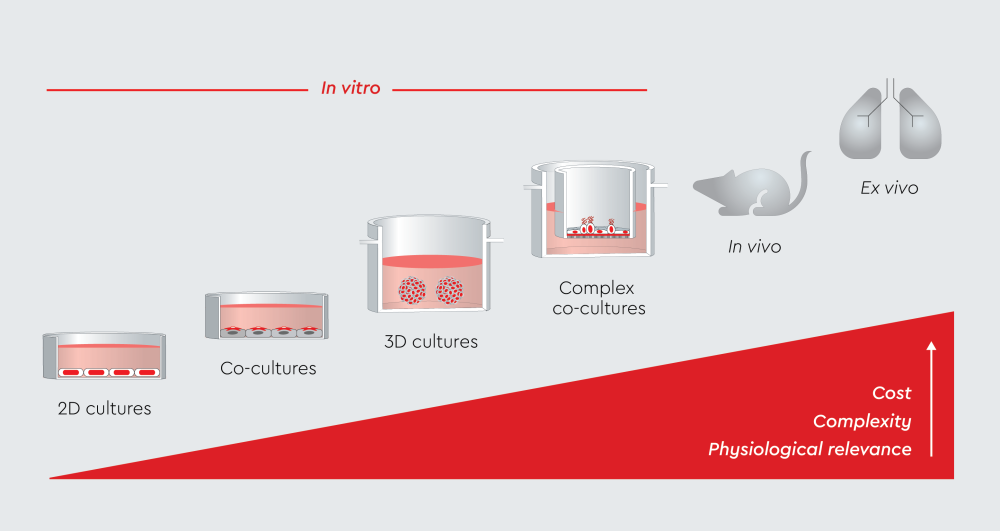

Figure 1: Progression of in vitro cell culture models from basic 2D monolayers to physiologically relevant systems.
Air-liquid interface (ALI) cultures bridge the gap between simple in vitro systems and physiologically relevant models. They recreate the native airway environment where cells are exposed to air on the apical surface and culture medium on the basolateral side.
What is air-liquid interface culture, and why use it for airway epithelial cells?
Air-liquid interface culture is a technique in which epithelial cells grow with their apical surface exposed to air while their basal surface receives nutrients from the culture medium below.1 This configuration promotes a three-dimensional, differentiated epithelium that closely resembles the human airway.
The culture system typically uses cell culture inserts with permeable membranes that create two chambers. Primary human bronchial epithelial cells are seeded and, under ALI conditions, differentiate into a pseudostratified epithelium containing ciliated and goblet cells with proper polarity and specialized functions.1,5 In contrast, traditional 2D monolayer cultures often lose tissue-specific architecture and cellular specialization.6
Key features of ALI culture include1,3
- Tight junction formation between adjacent cells
- Mucociliary differentiation with functional cilia
- Barrier function measured by transepithelial electrical resistance (TEER)
- Mucus production by goblet cells
- Cell polarization with distinct apical and basal surfaces
How does air-liquid interface culture work?
The ALI culture process typically spans three to four weeks and involves five main stages.
- Initial seeding: Researchers seed primary cells onto plastic culture vessels where they expand until reaching 70%-90% confluence.
- Transfer to permeable membranes: Cells are transferred to compartmentalized culture inserts at a density of 150,000 living cells per cm².
- Submerged growth phase: For the first few days, cells remain fully immersed in culture medium on both apical and basal chambers. This allows proliferation and initial monolayer formation.
- Air-lifting: Once cells are confluent, the apical medium is removed, exposing the upper surface to air. The basal chamber continues to provide nutrients through the permeable membrane.
- Differentiation period: Over 2-3 weeks, cells undergo morphological and functional differentiation. The culture system exhibits pseudostratified architecture, ciliated cells, mucus-producing goblet cells, and tight junctions.
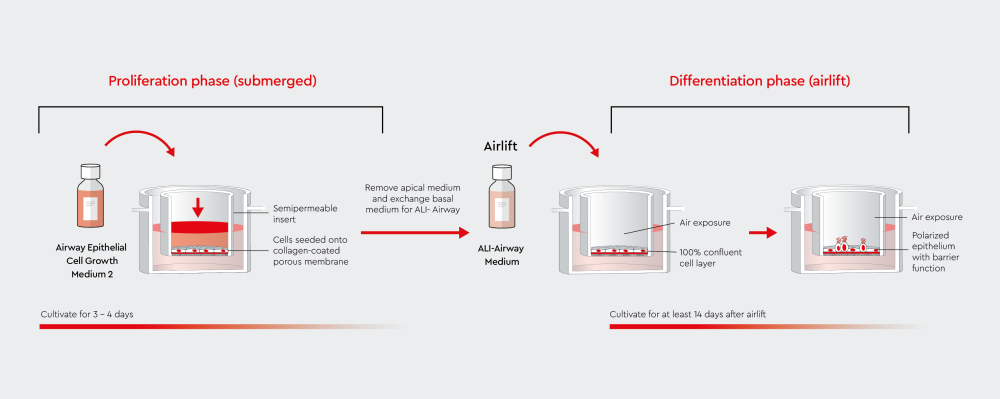

Figure 2: Air-liquid interface (ALI) culture workflow comparing submerged and airlifted conditions.
In submerged culture, airway epithelial cells are covered by medium in both apical and basal chambers. During airlift, the apical surface is exposed to air while the basal chamber continues to provide nutrients. This exposure induces cell polarization and differentiation, forming a functional airway epithelium with barrier properties.
Advantages of air-liquid interface (ALI) culture systems for respiratory research
Air-liquid interface culture offers several advantages over conventional 2D cell culture systems. These advantages address key limitations of in vitro models for respiratory research.
Physiological relevance
ALI cultures maintain native airway architecture. Cells develop polarity, form tight junctions, and exhibit specialized functions such as mucociliary clearance.1 This contrasts with 2D cultures, in which cells often dedifferentiate and lose tissue-specific properties.
Barrier function
The development of tight junctions creates a barrier that can be measured using TEER values. ALI cultures can achieve TEER values above 500 Ω·cm², indicating strong barrier integrity.7
Cellular differentiation
Unlike cell monolayers, ALI systems support the development of numerous cell types, including ciliated cells, goblet cells, and basal cells.1 This cellular diversity more accurately represents the complexity of human airways.
Reduced animal testing
ALI culture systems align with the 3R principle (Replace, Reduce, Refine) by providing human-relevant models that can reduce dependence on animal testing.
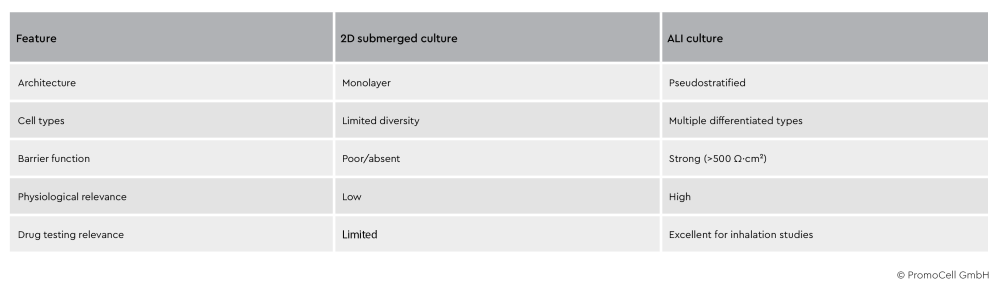

Table 1: Comparison of characteristics of 2D submerged cultures and ALI cultures
By exposing airway cells to air on the apical surface, ALI cultures restore key features such as tight junctions, mucus production, and barrier integrity. This allows for increased reliability of in vitro airway studies.
Applications of ALI culture in research
Air-liquid interface culture systems are versatile tools with applications across multiple areas of respiratory research.
Respiratory disease modeling
ALI cultures have been used to model chronic respiratory conditions including asthma, COPD, and cystic fibrosis.8 Owing to their physiological relevance, ALI systems can be used to study disease-specific changes in barrier function, inflammation responses, and cellular dysfunction.9 In addition, primary cells from patients with specific conditions can be cultured to create personalized disease models.10
Infectious diseases
The COVID-19 pandemic highlighted the need for human airway models for studying viral infections. ALI cultures have been used to study SARS-CoV-2, influenza, and respiratory syncytial virus (RSV).11 These systems allow researchers to characterize viral replication and host immune responses and to test antiviral compounds.
Environmental and toxicology studies
Air pollution, cigarette smoke, and industrial chemicals can be directly applied to the apical surface of ALI cultures, mimicking how environmental pollutants come in contact with the lungs. This approach allows researchers to understand how environmental chemicals contribute to respiratory diseases and to evaluate the safety profiles of new chemicals.12
Drug discovery and development
ALI culture represents the gold standard for testing aerosolized medications and inhalation therapies.1,13 Drugs can be applied to the apical surface as they would be delivered to patients, providing more clinically relevant data than traditional submerged cultures.
Personalized medicine
Using patient-derived primary cells, researchers can create patient-specific ALI models to test drug responses and study disease progression.1 This approach can support the development of personalized treatments for respiratory diseases.
Limitations of ALI culture
Despite their advantages, ALI culture systems also present technical and practical challenges that researchers may have to address.
- Technical complexity: ALI cultures require equipment that may not be available in all labs. This includes cell culture inserts, incubators with precise environmental control, and TEER measurement devices. The multi-step protocol for developing ALI cultures requires technical expertise and may need to be optimized.
- Time and cost considerations: Cells in ALI cultures take 3-4 weeks to fully differentiate. This extended timeline increases costs and requires long-term experimental planning.
- Donor variability: Primary human cells show inherent biological variation between donors. Factors such as age, health status, medication history, and genetic background can all influence the characteristics of ALI cultures when primary cells are used.14 Some donors may have compromised barrier function, particularly those with chronic respiratory conditions.15
- Reproducibility across laboratories: Different research groups may use different protocols, media formulations, and culture conditions, which could affect the performance of ALI cultures.5,16 This variability makes it difficult to compare results across studies.
Solutions and best practices
Common challenges in ALI cultures include contamination, uneven differentiation, and loss of tight junction integrity. Precautions and troubleshooting may be required to address these issues.
- Standardized media: Serum-free and BPE-free formulations reduce variability and improve experimental reproducibility.
- Pre-screened cells: Quality-controlled primary cells that have been evaluated specifically for ALI culture performance.
- Quality control: Regular TEER measurements and morphological assessments to ensure culture quality.
- Standardized protocols: Following established, validated procedures to improve reproducibility.
How we support your respiratory research
At PromoCell, we are committed to advancing air-liquid interface research by providing high-quality products.
- Airway Epithelial Cell Growth Medium 2: A serum-free and BPE-free formulation optimized for the in vitro cultivation of epithelial cells from large air passages. This medium complements our complete BPE-free system when combined with our ALI-Airway medium.
- Air-Liquid Interface Medium (ALI-Airway): Our specialized ALI medium is a serum-free and BPE-free formulation for an optimal and standardized culture of human bronchial epithelial cells at the air-liquid interface.
- Airway Epithelial Cell Growth Medium XF (prf): Xeno- and serum-free cell culture medium for expansion of epithelial cells isolated from large airways. Also available as a GMP-grade formulation throughout our Custom GMP Services.
- ALI-pre-screened HBEpC with verified donor metadata: We provide quality-controlled cells validated for ALI performance, including TEER measurements and anonymized donor details such as age, sex, health background, and available HLA information.
We also provide expert scientific support to assist you in establishing and troubleshooting ALI cultures.
Future directions in ALI culture
Researchers continue to advance air-liquid interface culture by integrating new technologies and specialized applications.
- Organ-on-chip integration: Microfluidic "lung-on-chip" devices combine ALI culture with mechanical forces and flow, better replicating the physiological environment of the lungs.17,18
- Co-culture systems: Advanced ALI models now incorporate immune cells and fibroblasts to create more complete tissue representations.1,19 These co-cultures better model inflammatory responses and tissue interactions.
- Patient-derived models: The use of cells from patients with specific diseases or genetic variations allows researchers to create personalized ALI models.20
- Regenerative medicine applications: ALI culture techniques are being adapted for tissue engineering applications for the development of therapeutic interventions for severe lung diseases.21,22
To learn more about the emerging role of ALI culture in regenerative medicine, read the interview with our expert Dr. Elfie Rödel.
Conclusion
Air-liquid interface culture is advancing respiratory research by providing researchers with physiologically relevant in vitro models that offer a middle ground between monolayer cell cultures and animal studies. The technology continues to evolve with higher standardization, better-defined culture media, and integration with emerging platforms like organ-on-chip devices. As respiratory diseases, environmental pollution, and respiratory pathogens continue to challenge human health, ALI culture systems become an essential tool for respiratory research.
Advance your respiratory research with our latest airway culture solutions
Discover the new Airway Epithelial Cell Growth Medium 2, formulated to support consistent differentiation and reproducible ALI performance.
References
Expand
- Baldassi D, Gabold B, Merkel OM. Air−liquid interface cultures of the healthy and diseased human respiratory tract: promises, challenges, and future directions. Advanced NanoBiomed Research. 2021;1(6):2000111. doi:10.1002/anbr.202000111
- Chen S, Schoen J. Air-liquid interface cell culture: From airway epithelium to the female reproductive tract. Reproduction in Domestic Animals. 2019;54(S3):38-45. doi:10.1111/rda.13481
- Wu J, Wang Y, Liu G, et al. Characterization of air-liquid interface culture of A549 alveolar epithelial cells. Braz J Med Biol Res. 2018;51(2):e6950. doi:10.1590/1414-431x20176950
- Tam A, Wadsworth S, Dorscheid D, Man SFP, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5(4):255-273. doi:10.1177/1753465810396539
- Leung C, Wadsworth SJ, Yang SJ, Dorscheid DR. Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2020;318(5):L1063-L1073. doi:10.1152/ajplung.00190.2019
- Li Y, Kilian KA. Bridging the gap: From 2D cell culture to 3D microengineered extracellular matrices. Advanced Healthcare Materials. 2015;4(18):2780-2796. doi:10.1002/adhm.201500427
- Leiby KL, Raredon MSB, Niklason LE. Bioengineering the blood-gas barrier. Compr Physiol. 2020;10(2):415-452. doi:10.1002/cphy.c190026
- Shrestha J, Paudel KR, Nazari H, et al. Advanced models for respiratory disease and drug studies. Medicinal Research Reviews. 2023;43(5):1470-1503. doi:10.1002/med.21956
- Choi KYG, Wu BC, Lee AHY, Baquir B, Hancock REW. Utilizing organoid and air-liquid interface models as a screening method in the development of new host defense peptides. Front Cell Infect Microbiol. 2020;10. doi:10.3389/fcimb.2020.00228
- Pezzulo AA, Starner TD, Scheetz TE, et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2011;300(1):L25-L31. doi:10.1152/ajplung.00256.2010
- Michi AN, Proud D. A toolbox for studying respiratory viral infections using air-liquid interface cultures of human airway epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2021;321(1):L263-L279. doi:10.1152/ajplung.00141.2021
- Abo KM, Aja JS de, Lindstrom-Vautrin J, et al. Air-liquid interface culture promotes maturation and allows environmental exposure of pluripotent stem cell–derived alveolar epithelium. JCI Insight. 2022;7(6). doi:10.1172/jci.insight.155589
- Lenz AG, Stoeger T, Cei D, et al. Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air–liquid interface conditions. Am J Respir Cell Mol Biol. 2014;51(4):526-535. doi:10.1165/rcmb.2013-0479OC
- Nieuwenhuis TO, Giles HH, Arking JVA, et al. Patterns of unwanted biological and technical expression variation among 49 human tissues. Laboratory Investigation. 2024;104(6). doi:10.1016/j.labinv.2024.102069
- Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130(5):1087-1096.e10. doi:10.1016/j.jaci.2012.05.052
- Barosova H, Meldrum K, Karakocak BB, et al. Inter-laboratory variability of A549 epithelial cells grown under submerged and air-liquid interface conditions. Toxicology in Vitro. 2021;75:105178. doi:10.1016/j.tiv.2021.105178
- Kumar V, Madhurakkat Perikamana SK, Tata A, et al. An in vitro microfluidic alveolus model to study lung biomechanics. Front Bioeng Biotechnol. 2022;10. doi:10.3389/fbioe.2022.848699
- Thompson CL, Fu S, Heywood HK, Knight MM, Thorpe SD. Mechanical stimulation: a crucial element of organ-on-chip models. Front Bioeng Biotechnol. 2020;8. doi:10.3389/fbioe.2020.602646
- Mallek NM, Martin EM, Dailey LA, McCullough SD. Liquid application dosing alters the physiology of air-liquid interface (ALI) primary human bronchial epithelial cell/lung fibroblast co-cultures and in vitro testing relevant endpoints. Front Toxicol. 2024;5. doi:10.3389/ftox.2023.1264331
- Lo Cicero S, Castelli G, Blaconà G, et al. L1077P CFTR pathogenic variant function rescue by Elexacaftor–Tezacaftor–Ivacaftor in cystic fibrosis patient-derived air–liquid interface (ALI) cultures and organoids: in vitro guided personalized therapy of non-F508del patients. Respiratory Research. 2023;24(1):217. doi:10.1186/s12931-023-02516-0
- He ZJ, Chu C, Dickson R, Okuda K, Cai LH. A gel-coated air-liquid-interface culture system with tunable substrate stiffness matching healthy and diseased lung tissues. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2024;326(3):L292-L302. doi:10.1152/ajplung.00153.2023
- Campbell DR, Senger CN, Ryan AL, Magin CM. Engineering tissue-informed biomaterials to advance pulmonary regenerative medicine. Front Med. 2021;8. doi:10.3389/fmed.2021.647834
Related resources
