For more than a century, researchers who wanted to study human physiology, or discover and screen for new drugs had to rely on two-dimensional (2D) monolayer cells. However, when drugs or pathogens “look” for their target structures on the cells, they do not bump into the flat “full-on-display” structures found in 2D. Instead, they have to find their way through the intricate structure of the extracellular matrix (ECM), the complex tissue architecture, and the tight junctions that bind cells tightly together.

That is why cancer cells do not display some key features in 2D cultures – where they are much more sensitive to anticancer drugs – compared to 3D cell cultures, which better mirror the in vivo responses (Duval et al., 2017). When you are studying cell behavior and cellular processes, looking at monolayers can distort your results and ultimately prevent you from gaining valuable insights into what is really happening in the human body.
But what does growing cells in 3D mean for a researcher? And which method suits your purposes best? Our look at the history and uses of various cell culture techniques might help you decide whether switching from 2D to 3D fits your purpose. The technique has been around longer than you might think. Early mentions of “three-dimensional” cell culture showed up in scientific literature at the end of the 1980s. However, the origin of 3D cell culture arose at the beginning of the 20th century, when Ross Harrison developed the hanging drop tissue culture for studying the origin of nerve fibers (Harrison, 1906).
The classic: 2D cell culture
For most of the past century, 2D cell culture systems were the standard for many different applications, ranging from basic research to stem cell and cancer research to regenerative medicine. They are very well known, inexpensive, easy to analyze, and results can easily be compared to previous observations.
In the 1930s, scientists discovered that collagens were a universal component of connective tissue. This insight led to a series of experiments aimed at recreating scaffolds for the cells to grow in. In fact, 2D cell systems do not mimic very well the way cells grow and function in the body, because even coating of culture vessels with ECM proteins or synthetic polymers does not mirror the complexity of the in vivo architecture.
Before long, researchers at US materials-science company Corning developed MatrigelTM – a gelatinous protein mixture derived from mouse tumor cells (Kleimann and Martin, 2005), which made it possible to grow complex cellular networks under laboratory conditions. Complex networks were needed because adherent cultures usually only contain one type of cell. This means they do not allow the study of mixed cell populations that influence each other via cell-cell contacts and paracrine signals. Three-dimensional structures, in contrast, allow cells to interact with each other and with the matrix. These interactions greatly influence multiple cellular features such as vitality, proliferation, differentiation, migration, protein expression, gene expression, and response to stimuli (Baker and Chen, 2012).
The elevated interest in stem cell biology prompted researchers to come up with models that could faithfully replay the in vivo development of such cells. Yet morphological changes of cells and the subsequent functional alterations in 2D cultures were hurdles in scientists’ race to study the differentiation potential of stem cells. The shape of bone marrow mesenchymal stem cells, for example, directly influences their differentiation into osteoblasts or adipocytes. A round shape promotes adipogenic differentiation, yet cells with high spreading prefer an osteoblast fate. (Killian et al.,2010).
Although shape is significant, tissue architecture also plays a pivotal role. One example is in the study of cancer cell survival under different conditions, such as in a drug screening test. Cells in monolayers have the same unlimited access to nutrients and growth factors. But in vivo, the cell’s position inside a tissue determines whether it can get enough nutrients. (Kapałczyńska et al., 2018 ).
In response to the downsides of monolayer cultures, biomedical companies have developed various types of 3D cell culture system in the past decade, and scientists are quickly discovering the potential. Using more realistic representations helps researchers to better predict drug response in vivo (D’Aiuto et al., 2018 ). They also allow scientists to use fewer animal models. Three-dimensional cell culture techniques are continually improved and refined, however, one of the future challenges is to adapt cell-based assay protocols developed for 2D cell culture analysis to the altered conditions of cells growing in 3D structures.
Growing complexity: from 2D to 3D cell culture and organoids
Although having a variety of 3D systems is a good thing, it can produce uncertainty, as currently no universal 3D platform exists. In fact, when moving to 3D cell culture, one of the hardest steps might be choosing the technology that best suits your scientific investigation. Researchers are faced with many options including multicellular spheroids, scaffold hydrogels, organoids, organs-on-chips, hanging drop, microfluidics, magnetic levitation, microtissues, and 3D bioprinting (see infographic).
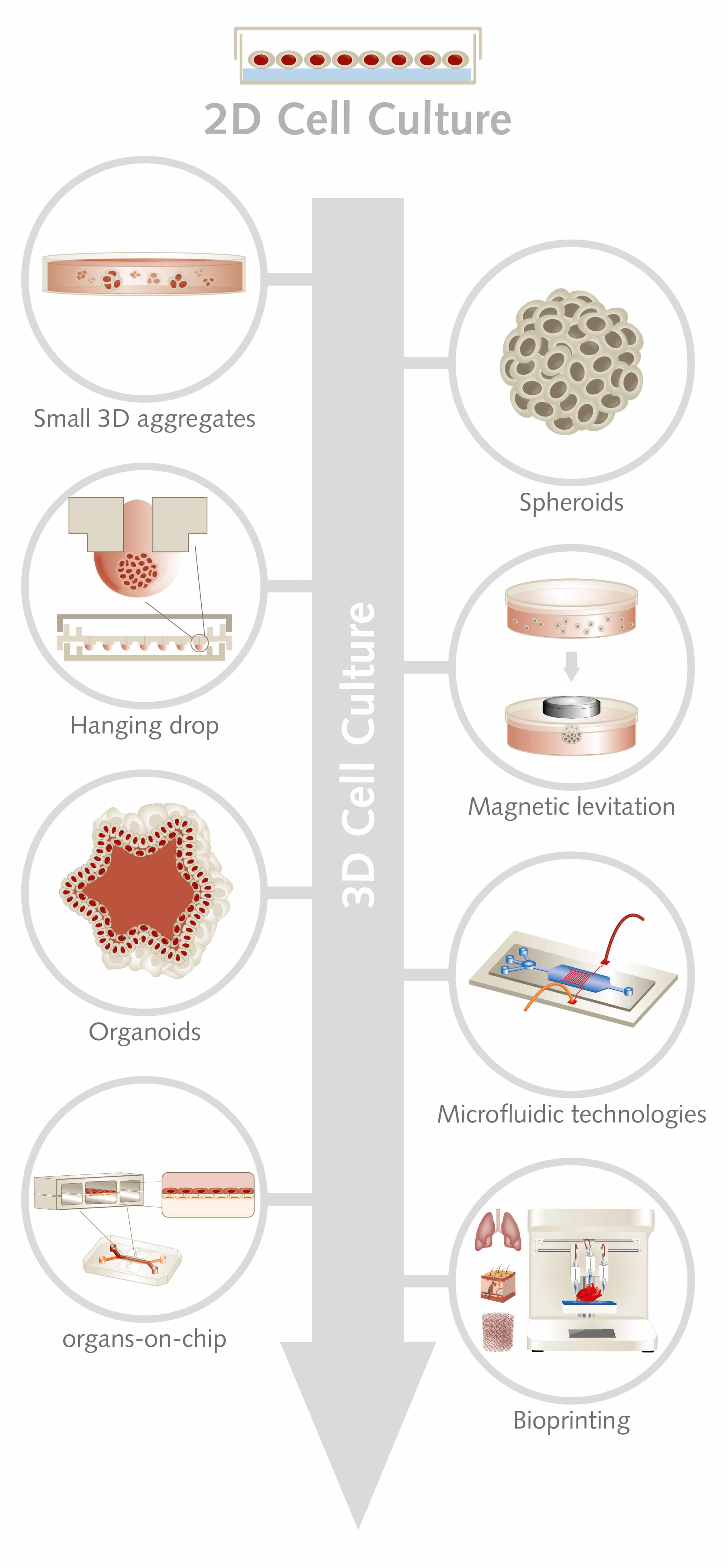
Designing large 3D cell constructs while maintaining cell viability remains a challenge, so small 3D aggregates with 500 to 10,000 cells represent a potential solution. The so-called microtissues can be used for cell culture purposes and as therapeutic agents, for cardiovascular regeneration, for instance (Günter et al., 2016).
Three-dimensional spheroids are one of the most simple and common way to culture cells in 3D and are used particularly in cancer research and regenerative medicine. Spheroids mimic, for example, tumor aggregates and can give valuable insights into the world of cancer stem cells and in their cancer-initiating properties. Spheroids can also be used to screen efficacy of anticancer drugs, as they provide biological relevant data by modeling the poor drug penetration in the center of the tumor mass (Zanoni et al., 2016). To induce spheroid formation, you can choose between methods such as suspension culture, nonadherent surface methods, hanging drop methods, and microfluidic methods.
The hanging drop technique is one of the simplest and most cost-effective methods, as it requires no specialized equipment or reagents. Here, a cell suspension is placed in a microwell, which is then inverted. A droplet forms where cells aggregate, creating compact and homogeneous spheroids (Kelm et al., 2003). However, spheroids might not be the method of choice when you need a large-scale expansion of cells, as it is difficult to control the size of the aggregates. Cells can agglomerate, leading to necrosis or apoptosis processes and to inhibition of cell proliferation (Bartosh et al., 2010).
For high throughput cell-based assays, scaffold-based 3D culture methods are often more suitable. They use 3D structures that allow cells to grow, proliferate, and differentiate in three dimensions on an extracellular matrix (ECM). Scaffolds are made of natural or synthetic biocompatible, and biodegradable materials with varying degrees of mechanical strength. There are two types of scaffold: rigid scaffolds, where cells are seeded in prefabricated structures, and self-assembling scaffolds, which form by encapsulating cells. The use of scaffolds helps scientists who are studying how the biochemical and biomechanical microenvironment influences cellular growth and differentiation, which is particularly important in stem cell research (see text box; Duval et al., 2017).
Another emerging technique for 3D tissue culture, which is suitable for high throughput screening, is magnetic levitation of cells in the presence of hydrogels. By controlling the magnetic field, the geometry of the cell mass and the clustering of different cell types can be manipulated to mimic gene expression, signaling and morphology of in vivo conditions (Souza et al., 2010).
If your aim is to mimic cellular processes in the original tissue, organoids might be the method of choice. Using stem cells – with their self-organizing properties – 3D structures with the key structural and functional features of gut, kidney, lung, brain and retina have been grown in the lab. With such models, scientists can study organ development, genetic diseases, inflammatory responses, and drug responses (Clevers, 2016). Also, tumor organoids can be established through ex vivo propagation of tumors from individual patients and used to screen anti-tumor drugs (Langhans, 2018).
With microfluidic technologies, scientists can manipulate of small volumes of fluids to control biological processes. This paves the way for studying the influence of different stimuli on the cellular microenvironment (Xiao et al., 2019). The use of microfluidics has led to the development of “organs-on-chip.” These are microchip devices containing living cells that are positioned in continuously perfused chambers to simulate tissue and organ function. Such devices allow researchers to study tissue development, organ physiology, and pathophysiology of diseases, as well as to discover and test new drugs (Bhatia and Ingber, 2014).
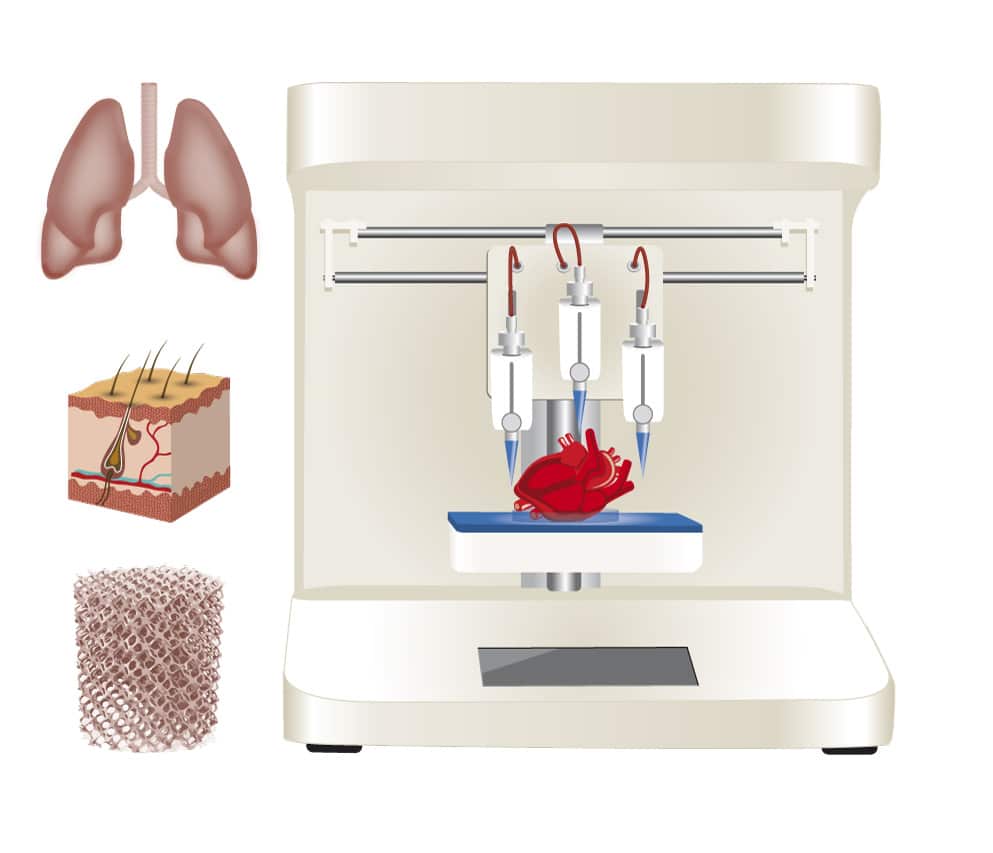
Three-dimensional bioprinting is a further evolution of 3D technologies. It allows the precise layer-by-layer construction of tissues and organs. With bioprinting, researchers can construct functional tissues such as vasculature, muscle, cartilage and bone, which can be used for drug discovery and preclinical testing (Huang et al., 2017).
Scaffold-based 3D cell culture vs. 3D bioprinting
In conventional 3D cell cultures, scaffolds made of different materials resemble the various tissue microenvironments. Cells are seeded manually, and you can choose between biological scaffolds that use materials such as MatrigelTM, BME, collagen, hyaluronic acid, alginate hydrogels and other biocompatible polymers.Highly automated 3D bioprinting creates precise and complex ECM-like scaffolds. This process layers cells, biological scaffolds and growth factors to create bioidentical tissues (Bishop et al., 2017). When deciding whether to use scaffold-based matrix or 3D bioprinting, consider your cell type and testing purpose. “Some cell types tolerate manual seeding better, as the stress level for them is lower than with bioprinting,” explains Dr. Jürgen Becker from PromoCell. “Yet 3D bioprinting is faster and creates a better reproducible generation of matrices with a more complex architecture.”
In 3D bioprinting, the cells are pre-positioned in the desired 3D structure, so there is no need to seed them. However, bioprinting still struggles with creating vascular networks that can support the cells with nutrients and remove waste. “Our 3D Scaffold Kit uses the same principle of 3D bioprinting,” says Becker. “A carrier scaffold, the alginate hydrogel matrix, is at the bottom of a microplate. After manual seeding of the cells, spheroids or organoids are built, which later can be isolated from the matrix.”
For high-throughput screening or tissue engineering, researchers tend to prefer automated approaches such as 3D bioprinting. Yet for lab-scale research, scaffold-based 3D cell culture is preferred, as it is less expensive with lower cell loss. Both techniques are vital for personalized regenerative medicine.
Growing cells in 3D: a challenging task
With so many possibilities, you may wonder whether moving from 2D to 3D is right for you. Most importantly, you want to make sure that tissue conditions are replicated accurately. Here are a number of factors you should consider before you begin. The design of the scaffold should support the development of an optimal ECM. It should also offer good control over the distribution of oxygen, carbon dioxide, nutrients and waste. Choose the microenvironmental factors based on their influence on cellular dynamics. Changing from the stiffness of the plastic surfaces of 2D culture dishes to the softness of ECM gels, for example, greatly affects cell adhesion, spreading, migration and differentiation.
Although 3D cell cultures provide realistic models of how cells assemble into tissues and organs, how tissues function, as well as how pathological processes disrupt cellular functions, they also present new variables. Three-dimensional models are more expensive and time-consuming than 2D, as few automation and reproducible applications are available. The 3D cultures also require careful planning and expert handling. You might find it difficult to get sufficient nutrients to the cells, as vascularization is missing. Additionally, you will have to develop new microscopic analysis workflows for the thick 3D structures, as it might be difficult to observe and measure cell processes with standard methods originally developed for 2D cell analysis.
One general problem of growing cells in 3D cultures is also the lack of standardization, which can make it hard to compare data (Duval et al., 2017). This is why scientists are working on developing standard and quick protocols, as well as reproducible 3D cell culture platforms, to be used in drug screening.
Deciding whether to use a 2D or 3D culture depends on your setting and research aim. In some cases, 2D studies are more feasible, quick, affordable, and can provide meaningful results. However, in other cases, you might consider 3D studies, as they yield more accurate results that can be better translated into clinical observations.