Animal component-free cell culture media
Researchers face increasing pressure to eliminate animal-derived components in cell culture while maintaining high cell growth. Animal component-free (ACF) formulations address concerns about batch variability and contamination risks, supporting robust cell growth while meeting regulatory and ethical requirements for cell culture.
What is animal component-free media?
Animal component-free cell culture media are specialized formulations that exclude any ingredients derived from animal or human sources. Instead, they contain inorganic salts, synthetic molecules, and defined proteins and peptides from non-animal origin, like E.coli or plants. This formulation results in a consistent, reproducible cell culture medium that is ready to meet both regulatory and ethical standards.
Raw materials are categorized based on their degree of processing and associated risk profile. Primary materials, such as fetal bovine serum (FBS), are directly sourced from animal origin. Secondary materials are derived from primary sources through intermediate processing steps. Bovine serum albumin (BSA) purified from FBS is an example of a secondary material substance. Tertiary materials refer to highly processed derivatives of secondary materials (e.g., amino acids derived from BSA), which typically present significantly reduced risk of adventitious agent transmission due to extensive purification and inactivation procedures.
Our ACF media are entirely free of animal and human components, even at the tertiary level. Even the recombinant proteins used are free from animal and human origins, ensuring maximum safety and ethical compliance.
Is chemically defined media the same as animal component-free?
Although both chemically defined and animal component-free media differ from traditional cell culture media, these are distinct formulations.
- Animal component-free media are completely free of both animal- and human-derived materials, offering a high level of safety and regulatory compliance.
- Chemically defined (CD) media go a step further by including only synthetic and inorganic components with known chemical structures and concentrations, ensuring maximum transparency and reproducibility. While both animal component-free and chemically defined media exclude animal-derived substances, CD media emphasize full chemical characterization.
- ‘‘Defined’’ formulations, on the other hand, include components with known identity and origin but may incorporate purified proteins or lipids from animal, human, or non-animal sources. All three media types are serum-free, eliminating the variability of undefined supplements and making them ideal for consistent, controlled research and regulated applications.
We at PromoCell also offer defined and/or animal component-free media, which are cell culture formulations composed entirely of components (raw materials) with known concentration and purity, excluding all animal- and human-derived substances to ensure consistency and safety.
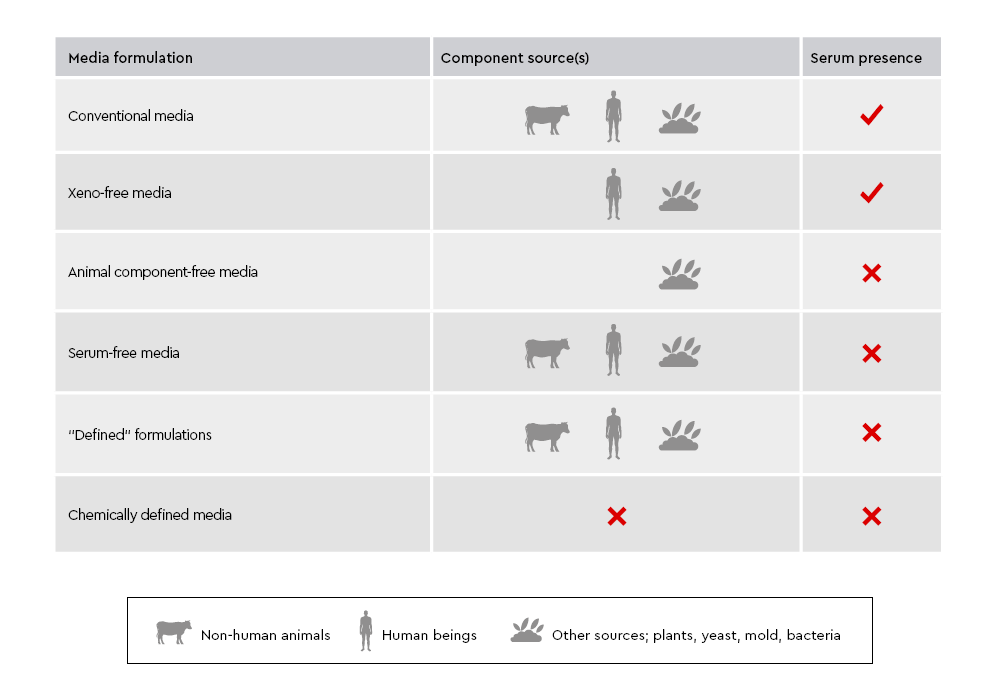

Figure 1: Comparison of component sources and serum presence between animal component-free, chemically defined, and defined cell culture media. Animal component-free media exclude all animal- and human-derived materials, while chemically defined media contain only components with known chemical structures. Defined media include components with known identity and origin but may contain purified materials from various sources.
Why are animal component-free media important?
Research methodology continues evolving toward more ethical and sustainable practices. The increasing adoption of 3D model systems, organ-on-chip technologies, and AI-driven approaches is reducing reliance on animal models. This shift requires transition to ACF media, which offer many advantages.

Applications in advanced cell therapies
Cell-based therapeutics, including research with induced pluripotent stem cell (iPSC)-derived cells particularly benefit from animal component-free formulations. During the early phases of therapeutic development, the quality of medium formulation and raw materials significantly influences cell growth, viability, differentiation, and ultimately, the final product quality. Getting these factors right from the start of development is key for later success.
Clinical development phases require reagents in cell therapy manufacturing that guarantee safety and product suitability. Early implementation of such raw materials reduces both developmental risks and downstream costs, making ACF media a smart long-term investment.
Performance of serum-containing media vs. animal component-free media
Serum-free workflows can seem like a big leap, but the improved control and reduced variability of ACF media make it a smart move for forward-looking labs. Our performance-matched ACF media are backed by data demonstrating support for complex applications without compromising quality.
Comparative studies show that cells cultured in ACF media achieve growth rates and morphological characteristics equivalent to those grown in conventional serum-containing formulations. Fibroblast cultures, for example, demonstrate identical expansion kinetics and maintain characteristic cellular morphology when grown in defined animal component-free conditions versus traditional serum-supplemented media.
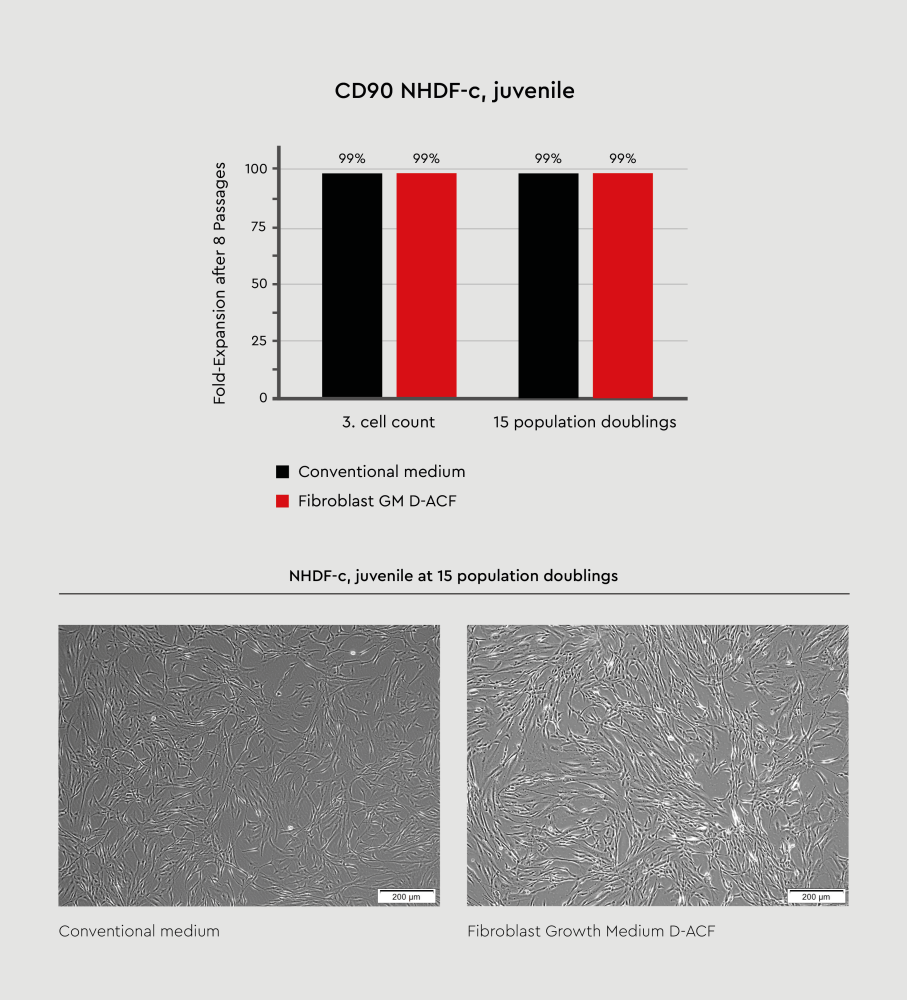

Figure 2: Growth of fibroblast grown in conventional serum-containing media versus those cultured in animal component-free formulation. Normal human dermal fibroblasts (NHDF), from juvenile donors, were cultured in either conventional Fibroblast Growth Medium or Fibroblast Growth Medium D-ACF under identical conditions. Cell growth was monitored over time, demonstrating equivalent performance between both formulations. Both media types support robust cell expansion with comparable growth kinetics as well as CD90 expression. This confirms that animal component-free formulations maintain the high-quality standards of conventional serum-containing media.
Our ACF media lot undergoes rigorous quality control testing equivalent to that conducted for conventional formulations. Testing protocols include growth-promoting activity and typical morphology maintenance. Additionally, all media lots are tested for microbial contaminants, including fungi and bacteria.
Making the switch to animal component-free media
Adopting animal component-free protocols represents a strategic switch that can improve research quality and regulatory compliance. Start by evaluating your current applications and identifying where ACF media can deliver immediate value in terms of consistency and safety. Implement these formulations in pilot studies first, allowing your team to build confidence with the protocols before broader adoption. Our dedicated technical support specialists will work closely with you during this transition, providing guidance on protocol optimization and troubleshooting to ensure seamless integration into existing workflows.
Our animal component-free solutions
Our ACF media portfolio empowers ethical, animal-free research, removing regulatory barriers and enabling you to focus on innovation. With the launch of animal component-free formulations, researchers can confidently transition to animal component-free workflows, moving closer to clinical applications with consistency, safety, and complete peace of mind.
Our growing collection of ACF media includes specialized formulations for various primary cell types. These products support research in regulated environments while maintaining experimental safety, consistency, and ethical compliance.
Beyond animal component-free
For applications that require the highest regulatory standards, our PromoExQ portfolio delivers Excipient GMP-grade formulations specifically designed for clinical and translational research. These advanced products meet stringent manufacturing requirements and provide the quality assurance necessary for therapeutic development programs and regulatory submissions.
