Xeno-free cell culture media
Cell culture scientists today face increasing pressure to develop therapies that meet stringent regulatory standards. Traditional media containing components of animal origin pose significant challenges for clinical applications. The development of cell therapy, in particular, relies on the use of safer, more reliable culture conditions. Xeno-free culture media minimizes contamination risks while maintaining optimal cell growth. In this guide we explore everything you need to know about xeno-free cell culture media and their role in biotechnology.
What does xeno-free mean in cell culture?
Xeno-free culture media are cell culture formulations that exclude non-human animal-derived components. Instead, they contain human-derived components or recombinant human proteins, extracted from human serum or produced in human cells / cell lines (E.g., HEKs).
The term xenogeneic-free refers to the absence of foreign animal materials that could introduce pathogens or trigger immune responses. Therefore, the use of xeno-free (XF) media in cell culture supports safer applications in regenerative medicine and cell therapy development.
What are examples of xeno-free culture media components?
XF media typically contain some of the following human-derived elements:
- Recombinant human proteins (growth factors, cytokines, attachment factors)
- Human serum albumin (HSA) for protein supplementation
- Human platelet lysate (hPL) as a serum replacement
- Human-derived extracellular matrix components
- Chemically defined supplements without animal origin
What are examples of xeno-free culture media components?
XF media typically contain some of the following human-derived elements:
- Recombinant human proteins (growth factors, cytokines, attachment factors)
- Human serum albumin (HSA) for protein supplementation
- Human platelet lysate (hPL) as a serum replacement
- Human-derived extracellular matrix components
- Chemically defined supplements without animal origin
Can xeno-free culture media include human serum?
XF media can include human serum, which provides nutrients and growth factors that are essential for cell growth while maintaining the xeno-free status.
What is the difference between animal-free and xeno-free?
The main distinction between animal-free and xeno-free culture media lies in their source of biological components. Xeno-free culture media may include materials derived from human sources but exclude non-human animal components. In contrast, animal-free media eliminate all components of animal origin, including those from humans. Animal-free media rely on non-animal-derived recombinant peptides (e.g., from bacteria, yeast, or plants) and synthetic components to support cell growth.
What’s the difference between xeno-free and serum-free media?
Many researchers use the terms "xeno-free" and "serum-free" interchangeably, but these terms describe media with different characteristics. Serum-free media means no serum from any animal or human source is present. However, other animal-derived or human-derived components can be included in serum-free formulations.
When a cell culture medium is labeled as both xeno-free and serum-free, it contains no human serum but may include other human-derived components. This combination offers the benefits of both approaches while maintaining defined conditions.
Key distinctions:
- Serum-free media: No serum present, but may contain other animal components
- ACF media: Exclude animal- and human- derived components
- Xeno-free serum-free media: No serum and no animal-derived components, but may include human-derived components other than serum
Understanding these differences enables researchers to select the most suitable media for their specific applications.
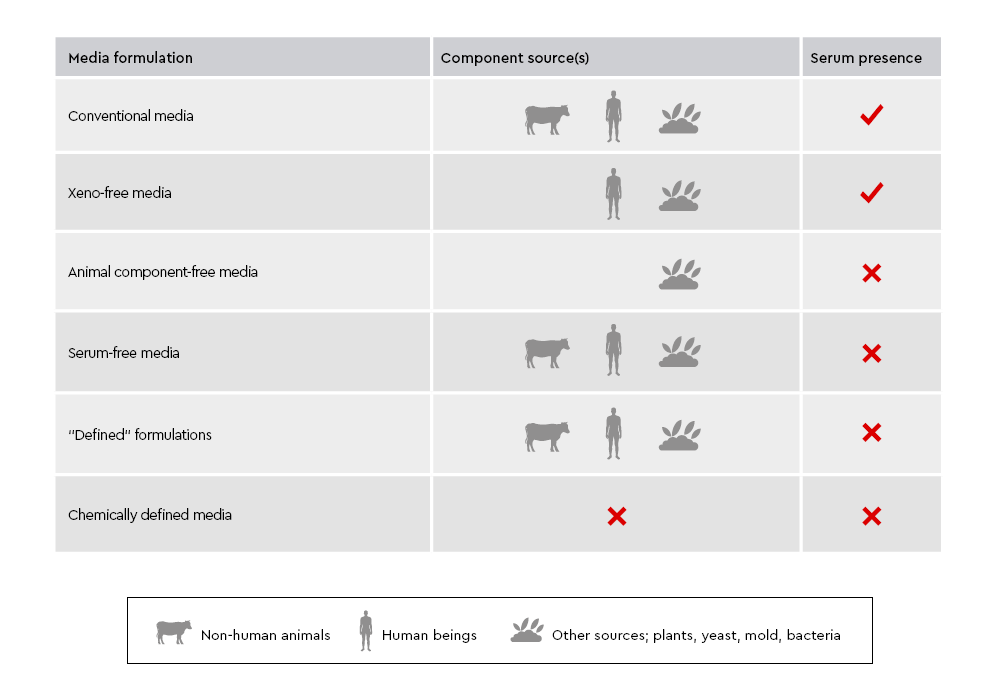

Figure 1: Comparison of cell culture media types. Xeno-free culture media exclude components from different species, while animal component-free media exclude all animal- and human-derived components. Chemically defined media contain only materials with known chemical structures, and defined media contain components with known identity and origin.
What are the benefits of xeno-free cell culture media?
XF media offers several advantages that make it essential for the development of cell therapies.
- Enhanced safety and regulatory compliance: XF media eliminates the risk of xenogeneic contamination from animal pathogens. The reduction in contamination risk enhances regulatory compliance when developing cell therapies.
- Improved reproducibility: Standardized xeno-free formulations provide more consistent results compared to variable animal serum batches. This reproducibility is essential when scaling up cell cultures for manufacturing of cell therapy products.
- Better regulatory acceptance: Regulatory agencies increasingly favor xeno-free systems for cell therapy applications. These media formulations align with guidelines for the production of clinical-grade cells.
- Cost-effective long-term solutions: While initially more expensive, XF media reduces downstream costs associated with regulatory hurdles and clinical trial complications.
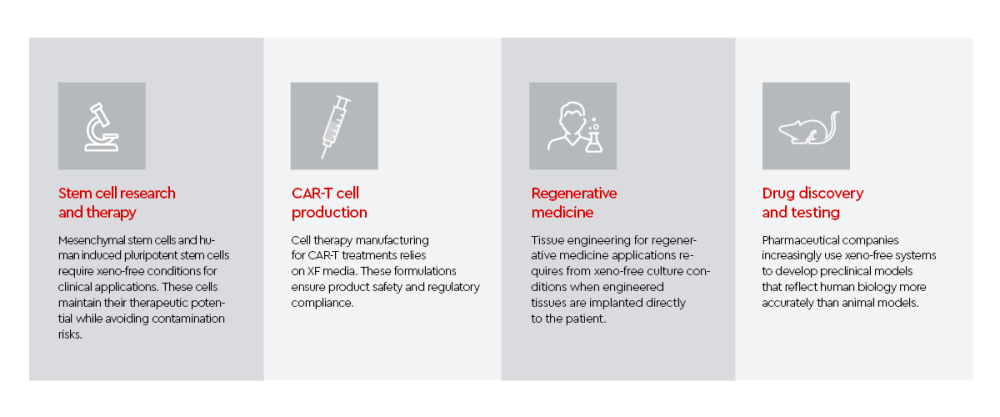
Applications of xeno-free cell culture systems
Xeno-free conditions have become standard in several therapeutic applications.

How can I transition to xeno-free culture?
Switching from traditional serum-containing media may require optimization of cell culture protocols to ensure optimal cell growth. XF medium can match the growth performance of serum-containing medium. However, simply removing serum without proper compensation could lead to poor cell growth. Xeno-free formulations require specialized components that replace the growth-promoting effects of serum.
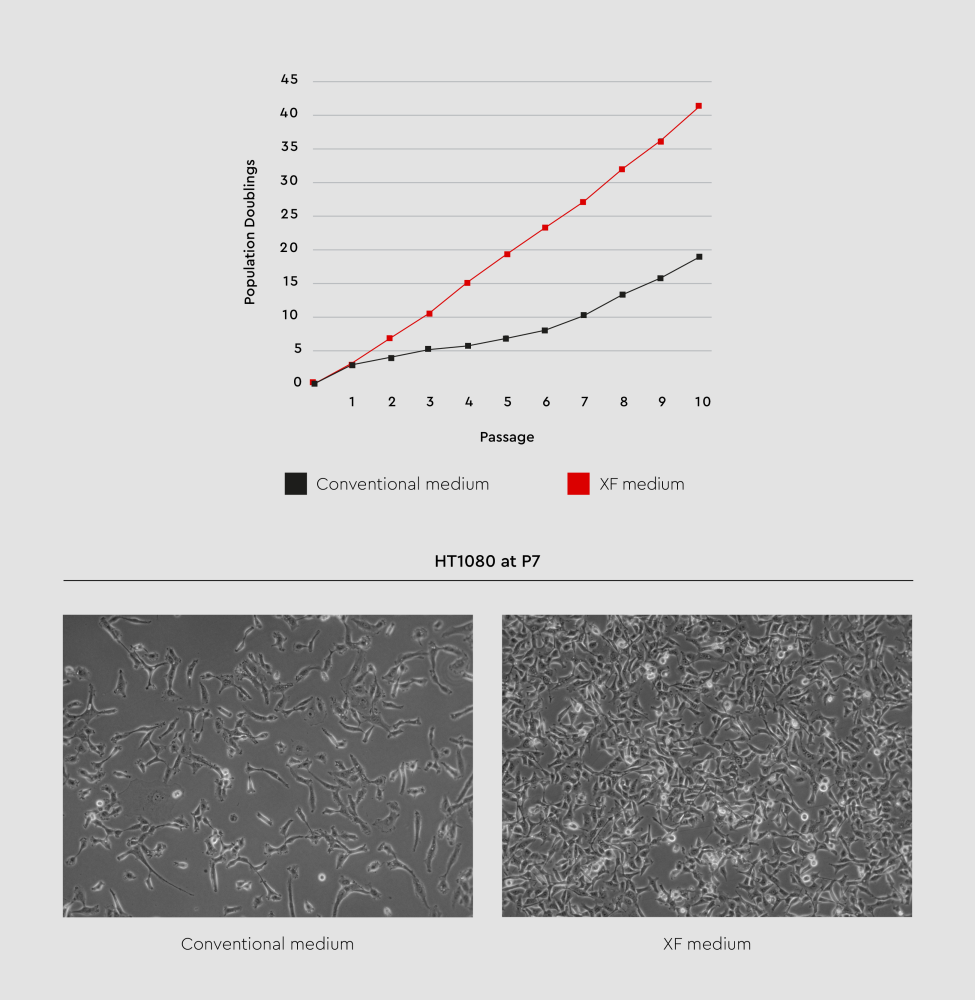

Figure 2: Growth performance comparison of HT1080 fibrosarcoma cell line between xeno-free media and conventional DMEM with FBS. XF media provide high performance in terms of promoting cell growth and maintaining cell morphology.
All XF cell culture media undergo rigorous quality control to ensure their high performance, following the same testing protocols used for conventional media. They also undergo testing to confirm the absence of microbial contaminants, including fungi and bacteria.
Plan for the transition
Ready to go xeno-free? With a bit of upfront planning, you'll set yourself up for long-term success. Begin by identifying applications where XF media can provide immediate benefits. Then, you can gradually expand the use of xeno-free formulations as comfort with the technology grows. Our scientific support team is here to guide you throughout the transition process, ensuring smooth protocol adaptation.
Our xeno-free solutions
We offer XF media formulations for various cell types and cell culture applications. Our specialized formulations support the growth of sensitive and primary cell types while ensuring consistent and reliable results. With comprehensive testing, our solutions empower translational and regulated research.
For advanced applications
If you need something more, our PromoExQ portfolio offers Excipient GMP-grade designed for clinical and regulatory applications. These products represent the next level of cell culture technology, providing the highest possible quality standards for the most demanding applications.
Our xeno-free solutions
We offer XF media formulations for various cell types and cell culture applications. Our specialized formulations support the growth of sensitive and primary cell types while ensuring consistent and reliable results. With comprehensive testing, our solutions empower translational and regulated research.
For advanced applications
If you need something more, our PromoExQ portfolio offers Excipient GMP-grade designed for clinical and regulatory applications. These products represent the next level of cell culture technology, providing the highest possible quality standards for the most demanding applications.
