Understanding our cell culture media formulations
Choosing a suitable culture medium is crucial for in vitro cell cultivation, affecting the success of cell culture experiments, drug development, and clinical translation. Beyond supporting optimal cell growth, media formulation plays a critical role in experimental planning and risk assessment processes.
Why a clear understanding of media formulation is important:
- When working in cell-based therapy development and regenerative medicine, the media formulation directly influences safety profiles and risk assessments. We provide a wide range of advanced media formulations to meet the requirements of researchers working with various primary cells.
- It is essential for researchers to have a clear understanding of the specifications of our cell culture media and reagents, from serum-free (SF) or xeno-free (XF) to animal component-free (ACF) or chemically defined (CD), to determine which formulation is most appropriate for different applications.
- It is also important to understand the specific properties of the media, such as phenol red considerations, intended use guidelines, available media formats (ready-to-use versus kits), and customization options to help you make informed decisions for your research and manufacturing processes.
How we define our media formulations
Animal component-free (ACF)
Our animal component-free (ACF) cell culture media are entirely free of both human- and animal-derived materials, offering maximum safety, consistency, and reproducibility. Suitable for various regulated applications, including cell therapy, biomanufacturing, and vaccine production, ACF media eliminate the risk of animal- or human-derived contaminants and enhance regulatory compliance. Fully aligned with risk management and GMP requirements, they provide a robust solution for high-quality, traceable, and regulatory-friendly cell culture processes.
Xeno-free (XF)
Our xeno-free (XF) cell culture media are formulated without any components from non-human animal sources, ensuring a safer and more consistent environment for cell growth, particularly in clinical and therapeutic applications. While they may contain human-derived materials such as human serum albumin (HSA), XF media eliminate the risks associated with animal-derived contaminants and variability, supporting compliance with regulatory requirements.
Designed for GMP processes, they support with strict risk assessments, offering a reliable solution for reproducible, high-quality cell culture in regulated environments.
Serum-free (SF)
Our serum-free (SF) cell culture media do not contain human or animal serum, offering consistent conditions to support reproducible cell growth and performance. Our serum-free media mitigate the risks associated with serum use, such as contamination and batch-to-batch variability. This makes them an excellent choice for biomanufacturing and research, supporting compliance with diverse requirements and aligning with risk management for safer, more reproducible processes.
Protein-free (PF)
Our protein-free (PF) cell culture media do not contain intact proteins (>50 amino acids, natural or recombinant) or serum preparations, reducing the complexity and variability associated with protein-containing media. Depending on the specific product, our PF media may contain peptides (<50 amino acids) and components of animal or human origin. Designed to simplify downstream processing and improve consistency, PF media are suitable for controlled biomanufacturing and research applications.
Chemically defined (CD)
Our chemically defined (CD) cell culture media contain only components of known chemical structure and concentration, ensuring maximum transparency, consistency, and control over cell culture conditions. Our CD products do not contain any substances of animal or human origin, and all ingredients are fully characterized. These formulations eliminate variability associated with undefined supplements. They are appropriate for high-precision applications that require streamlined regulatory compliance and risk management procedures.
Our CD products offer a reliable and reproducible solution for research, biomanufacturing, and GMP-regulated processes.
“Defined” formulations
Our defined-formulation cell culture media consist of components with known identity, concentration, and origin, ensuring maximum consistency and control over cell culture conditions. These media include purified recombinant proteins and peptides derived from human cells, other animals (e.g., insect cells or non-human mammalian cells), or non-animal sources. They may also contain well-characterized components such as purified human- or animal-derived lipids, plant-based ingredients, and semi-synthetic molecules from non-animal raw materials.
This precise composition eliminates the variability associated with undefined supplements, ensuring reproducibility, regulatory compliance, and robust risk assessments aligned with regulatory requirements. Therefore, defined formulations are ideal for controlled research, biomanufacturing, and GMP-regulated applications.
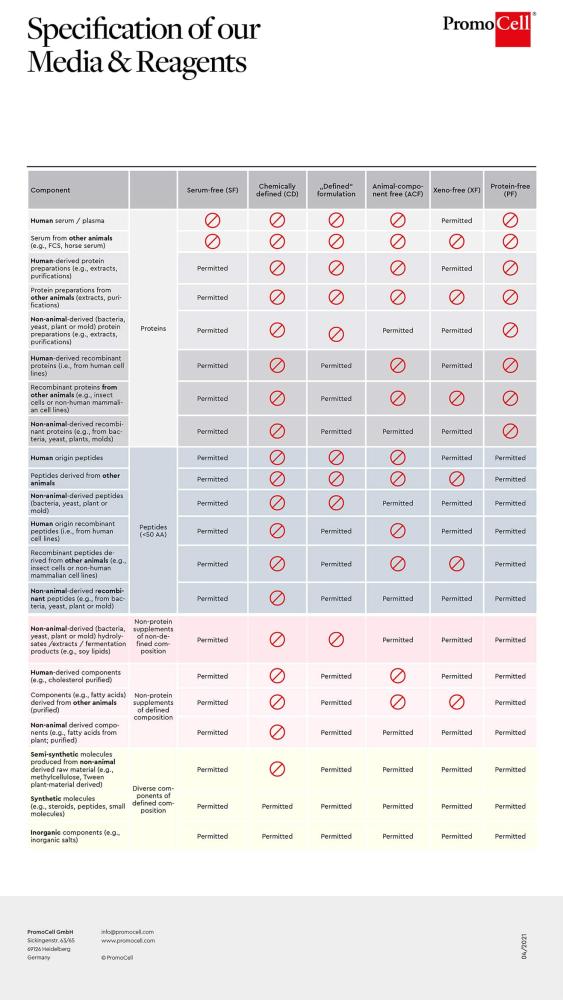
Which cell culture media components are allowed in different media specifications?
Building on the detailed definitions of our media formulations, we also provide a comprehensive visual overview of component compatibility across different media specifications. Our detailed specification guide offers an at-a-glance reference to help you quickly identify which components are permitted in each media type, supporting your decision-making process for optimal formulation selection.

When should phenol red be used in cell culture media?
When to consider phenol red in your cell culture media depends not only on your research goals but also on your risk assessment requirements. This pH indicator provides convenient visual monitoring of culture health and metabolic activity, changing from red (physiological pH) to yellow (acidic) or purple (alkaline) to signal potential contamination or nutrient depletion. However, the weak estrogenic properties of phenol red make it problematic for hormone-sensitive research, while its fluorescence characteristics can interfere with imaging and spectrophotometric assays.
Additionally, phenol red is often avoided in cell therapy and manufacturing applications due to the additional risk and safety concerns it introduces to the process. Phenol red-free cell culture media may be needed for hormone-dependent studies, fluorescence-based experiments, drug screening, stem cell research, and biopharmaceutical manufacturing.
Visit our blog post to learn more about the impact of phenol red on your research and explore our extensive range of phenol red-free solutions.
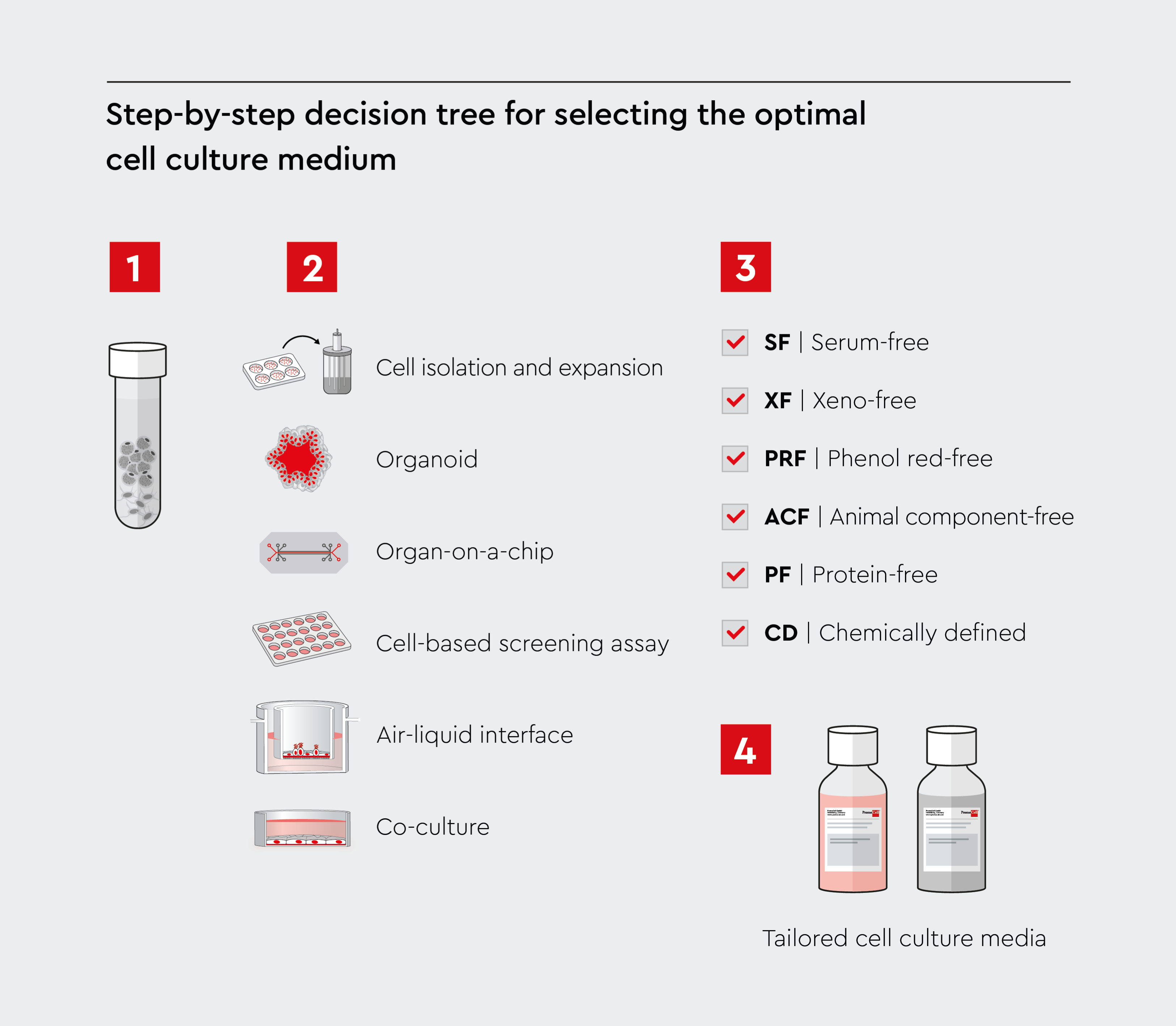
Choose the right cell culture media
Selecting the optimal cell culture medium requires a systematic approach that considers multiple factors.

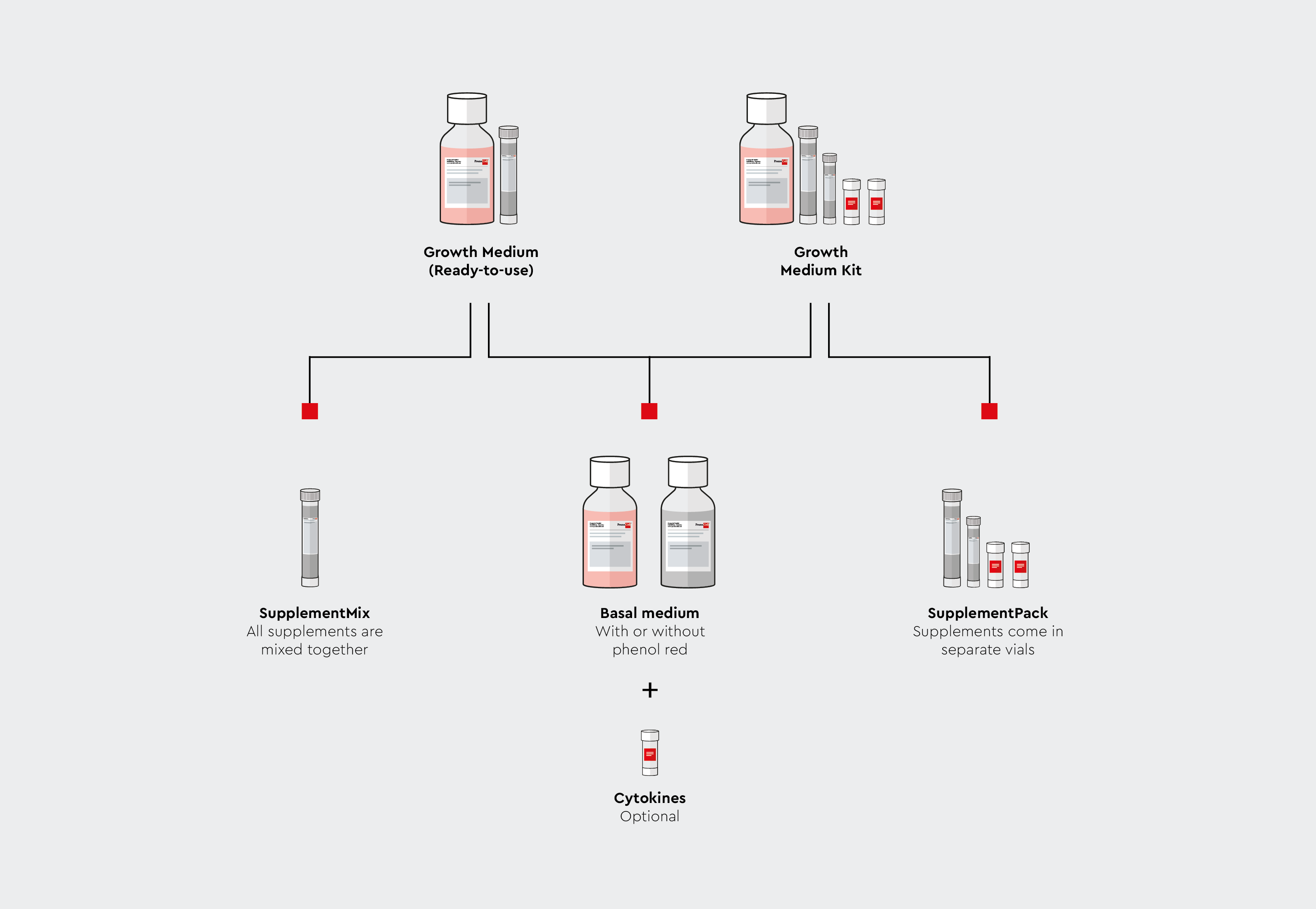
How are our media offered
We offer most of our cell culture growth media in two formats to suit your application needs.

Intended use of our products
Our standard research products are intended for in vitro research only. Our Excipient GMP-grade* and regulated media are intended for research use or further manufacturing. They are not intended for direct administration to humans or animals.
*‘GMP-grade’ is a branding term used by PromoCell to denote reagents that are manufactured at the PromoCell manufacturing facility in Heidelberg, Germany, under strictly controlled processes to meet stringent product specifications and customer requirements. Reagents manufactured at PromoCell are produced according to EXCiPACT™ GMP standards, a quality management system that builds on our ISO 9001:2015 certification. Risk assessment procedures are carried out at the customer site.
Customization of our media
We provide custom cell culture media formulations tailored to your needs, whether that involves special ingredients, specific testing requirements, or regulatory compliance. For larger projects, we offer upscaling and custom packaging options, including media bottles and bags of various sizes and bulk growth supplements. This flexibility allows you to focus on your research and further manufacturing, including transition to clinic and GMP processes, while we handle media production and compliance logistics.
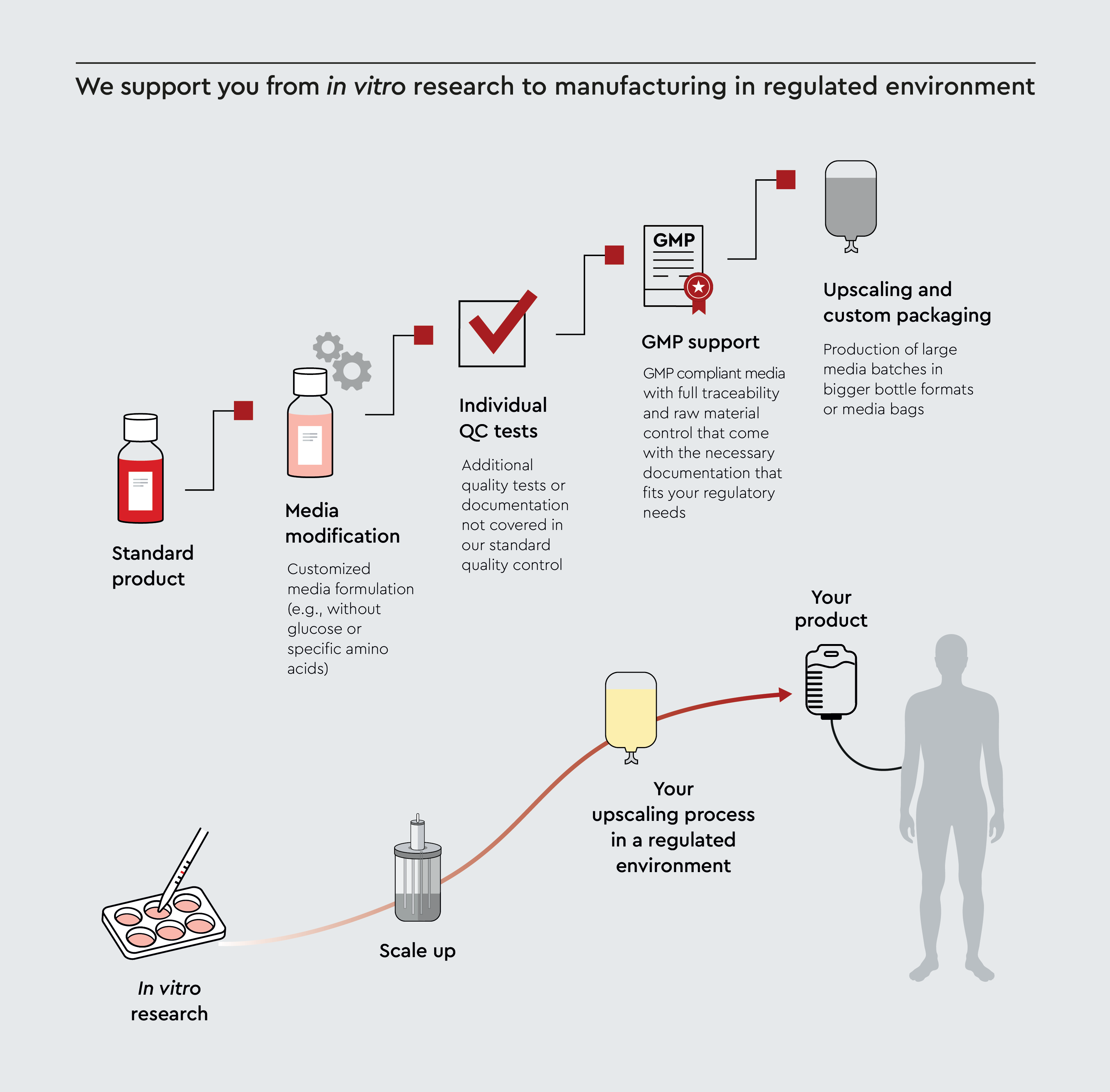

Figure 1: Our customizable media manufacturing process for biopharmaceutical applications. Starting from in vitro research and standard products, our cell culture media can be scaled up and customized through several quality-enhancing stages: media modification with customized formulations, individual QC tests for quality control, comprehensive GMP support with full traceability, and upscaling with custom packaging options.
FAQ
1. Can I access the ISO and EXCiPACT™ certification?
All our products meet the strictest European and international ethical standards, and our Quality Management System is certified according to ISO 9001:2015 and the EXCiPACT™ GMP standards. These certifications ensure that we consistently provide products and services that meet the requirements of researchers, applicable statutory and regulatory requirements, and GMP requirements according to NSF/IPEC/ANSI 363.
The following documentation can be directly downloaded from our website:
- Quality Policy Statement
- ISO 9001:2015 certificate
- EXCiPACT™ certificate
- ANSI 363-2016 certificate
2. Where can I find the Certificate of Analysis and the Material Safety Data Sheet?
Contact our Scientific Support to request the Certificate of Analysis (CoA) for our media & reagents: [email protected]
