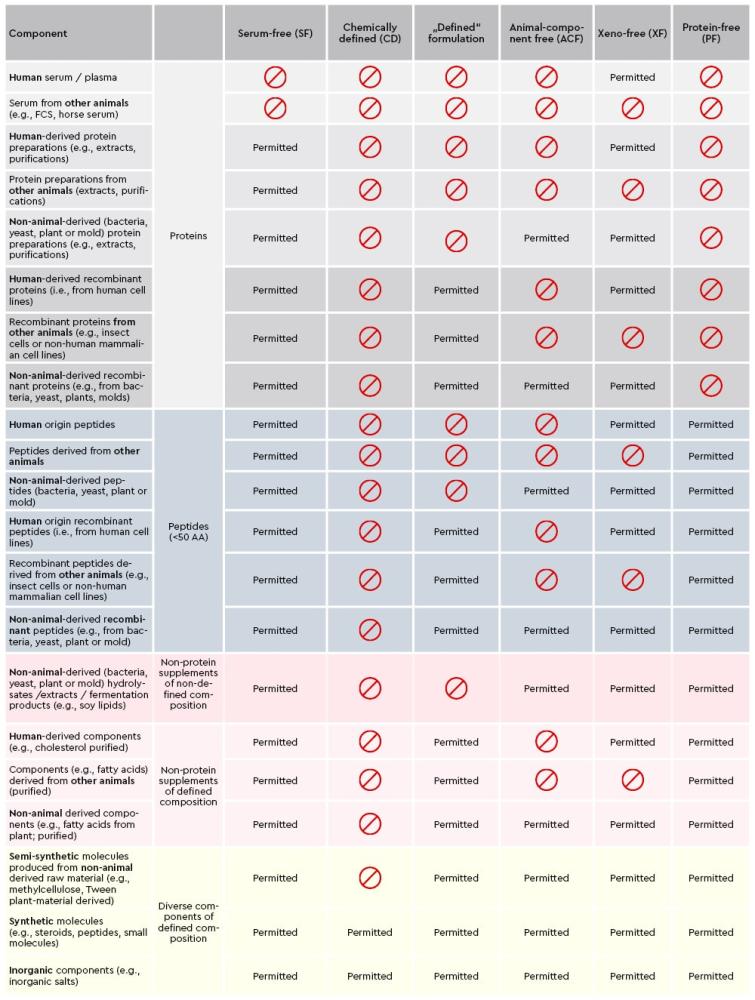
The integrity of the supply chain is a cornerstone of successful cell culture manufacturing. It directly influences product quality, regulatory compliance, and patient safety.
Through an interview with our experts, RegMedNet’s latest “In Focus” feature provides guidance on the requirements for maintaining GMP standards throughout the supply chain for cell culture products. The feature combines expert insights with practical advice to help you navigate the GMP-compliant supply chain management. It also provides strategies for risk mitigation, supplier qualification, and documentation practices. These practices are essential for maintaining product integrity from raw material sourcing to final delivery.
Infographic
This feature is accompanied by an infographic that provides a visual guide to ensuring GMP compliance in supply chain processes. The infographic outlines GMP requirements, from supplier qualification and raw material sourcing to storage, transportation, and distribution practices.
This practical resource serves as a quick reference for implementing robust supply chain management strategies.


Expert interview
Elena Cava Exposito, our Material Supply Manager, and Alica Tegethoff, our Project Engineer in Media Production, share their insights into GMP supply chain requirements in an interview with RegMedNet.
Our experts discuss various aspects of qualified supply chains and offer practical solutions to common production challenges.
The interview covers the following topics:
- The role of qualified supply chains in GMP cell culture products
- Most common GMP-related risks in today’s supply chains
- Best practices for assessing and qualifying suppliers to meet GMP requirements
- Managing changes in raw materials specification or suppliers while maintaining compliance
- Essential documentation practices for maintaining traceability throughout the supply chain
- The future evolution of GMP in supply chain management with digitalization and AI integration
This interview was made in collaboration with RegMedNet
Visit our feature Gain expert insights into qualified supply chains and discover practical solutions to streamline your production processes.