De-skilling 3D tumor models
Tissue-engineered 3D cancer models have grown in popularity with recent advances in cancer research. 3D models are more biomimetic than 2D cell monolayers cultured on tissue culture plastic. Examples are spheroids, tumorspheres, organoids, PDX and lately, tumoroids, which differ by origin (cell line or patient-derived), size, the complexity of cellular architecture and tumor environment.
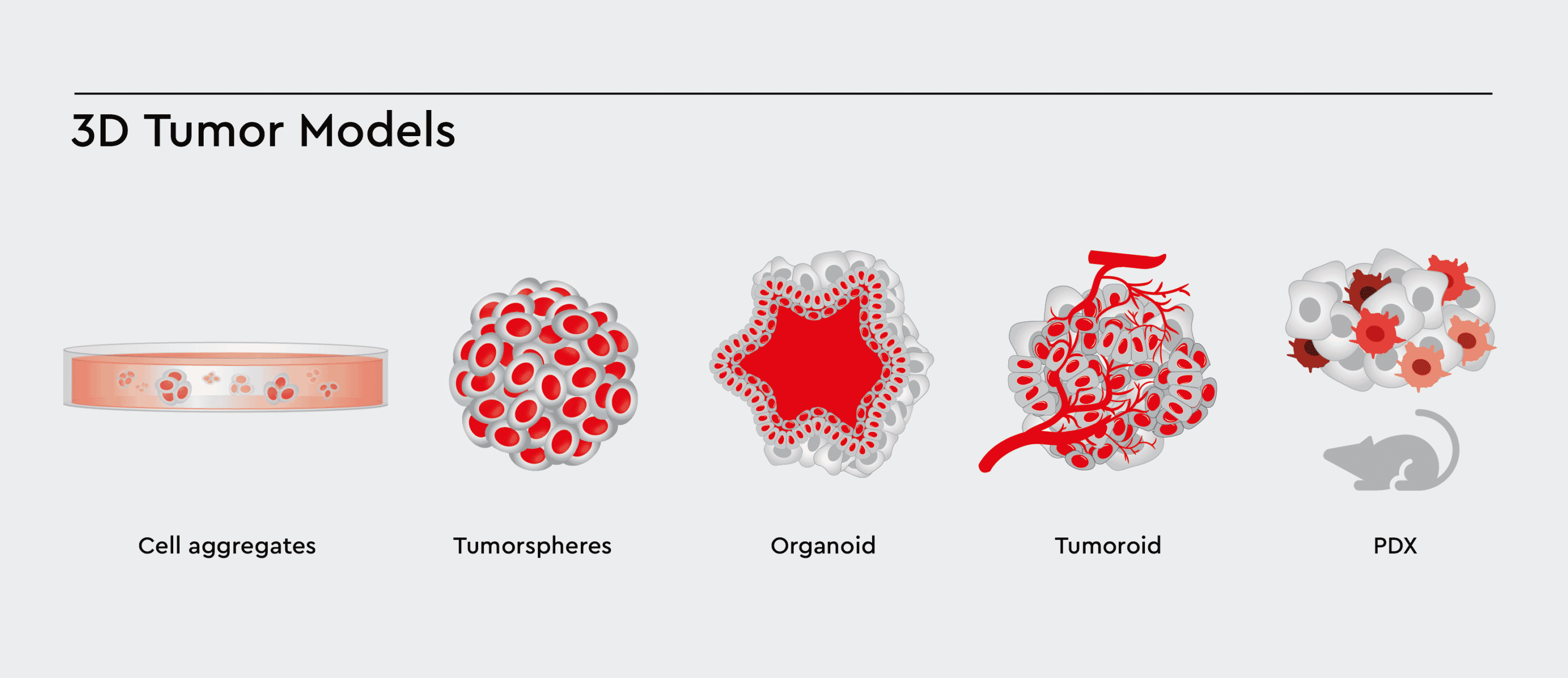
Figure 1: Various types of 3D Cell Models with increasing complexity. These 3D models aim to accurately model the tumor and tumor environment, allowing cells to cross-talk, differentiate, and migrate between the compartments.
Developing a suitable 3D tumor model can be pretty challenging, requiring special media, co-cultivation conditions and 3D support through i.g., scaffolds or extracellular matrix components. It is not only about mimicking the in vivo situation, but also the value of such a model stands and falls with its amenability to drug screening and drug testing.
One particular problem is the need for tumor models to contain a healthy cancer stem cell population to develop correctly. Not supporting the cancer stem cells causes two issues:
- 3D tumor models typically last for only a few days. Therefore, passaging and up-scaling are impossible.
- Tumorigenicity is lost (or at least reduced), which means that, e.g., efficient induction of tumors in PDX mouse models is not possible.
On the other hand, if you support cancer stem cells as a driver of tumor development, the tumor characteristics are automatically preserved, ensuring relevance to the in vivo situation.
Challenges in developing and using a 3D cancer model
- Obtaining the right starting material reflecting tumor biology and even individual patient-specific conditions
- Mimicking the cellular and matrix complexity of native tissues
- Ease and consistency of model to warrant assay reproducibility and to allow for up-scaling in screening assays
- increasing success when translating into in vivo models
- Defined and controllable experimental conditions
3D tumor models add substantial value to pre-clinical research and increase the successful translation from bench to clinic. However, to hold this promise, the models must be easy and robust enough to be employed in secondary assays and relevant for the in vivo situation. A healthy cell culture containing cancer stem cells is the essential aspect of receiving precisely this.
From cell line to 3D cancer model
Develop passageable 3D tumorspheres from any established cancer cell lines
We have developed a medium, 3D Tumorsphere Medium XF, that facilitates the steps from any starting material (established cell line or patient-derived biopsy) to the formation of functional tumorspheres.
Key advantages of the 3D Tumorsphere Medium XF:
- Tumorigenicity, as well as tumor characteristics, are maintained since CSCs are supported
- Universally applicable: many different cell lines or biopsies can be cultivated directly in the 3D Tumorsphere Medium XF
- Supports the creation of complex spheroid and organoids
- Reproducible formation of homogenous tumorspheres with spacial orientation
- Expansion and up-scaling of healthy tumorspheres that can be subcultured over 10 passages
- Efficient and low-cost ready-to-use kit with defined ingredients (3D Tumorsphere Medium XF is a synthetic growth medium)
The reason behind the reliable tumorsphere formation and their maintenance over an extended period and passages is the presence of CSCs. The CSCs carry over the tumor characteristics from the starting material and drive the tumor formation in culture as they do in vivo. The function of the 3D Tumorsphere Medium XF is to ensure a healthy native CSC population. Let biology do your job.
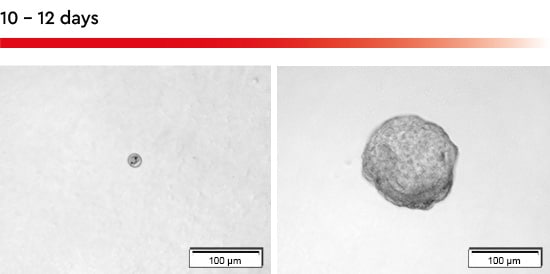
Figure 2: Tumorsphere formation with the 3D Tumorsphere Medium XF. Within 10-12 days a single cell seeded in an individual well of a 96-well u-bottom plate forms a tumorsphere with a size over 150 µm.
The 3D Tumorsphere Medium XF also supports non-CSC cancer cells to develop in a fully functional tumorsphere containing the spectrum of cancer cells with a different degree of differentiation. However, it eliminates, particularly in the beginning, non-cancer cells avoiding false cell aggregates. The result is clean tumorsphere populations within a single up to the complete multi-well plate.
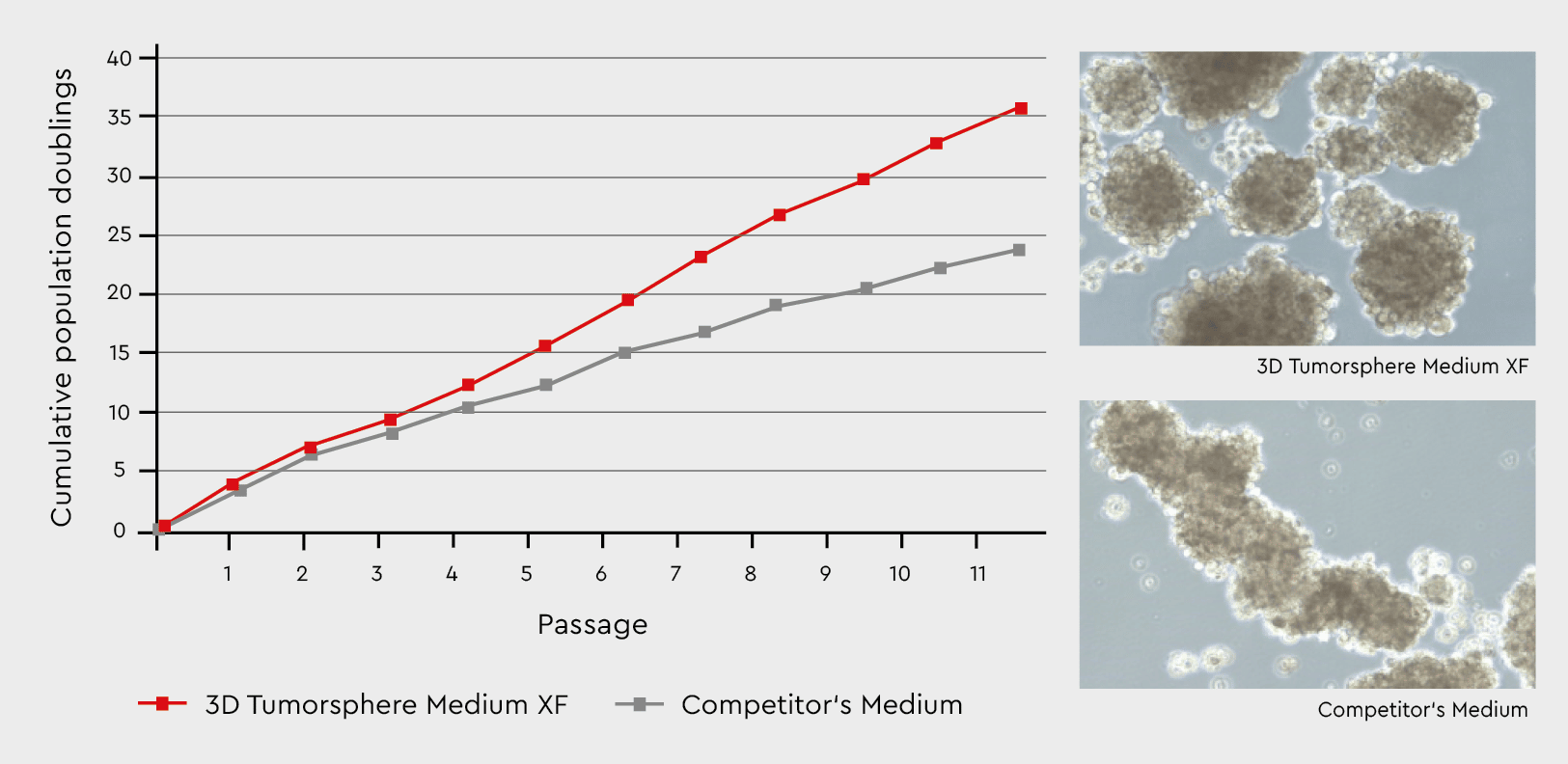
Figure 3: Tumorsphere formation and serial passaging in the 3D Tumorsphere Medium XF compared to a competitor’s medium. Developing tumorspheres in our 3D Tumorsphere Medium XF results in a significantly higher number of spheres due to the support of cancer stem cells. The medium leads to a consistent formation of homogenous tumorspheres.
From biopsy to 3D cancer model
Any easy way to go from biopsy to a meaningful 3D tumor model
The 3D Tumorsphere Medium XF supports sphere formation from established cell lines and biopsies. While the principal workflows are the same, the quality of biopsies is much more variable.
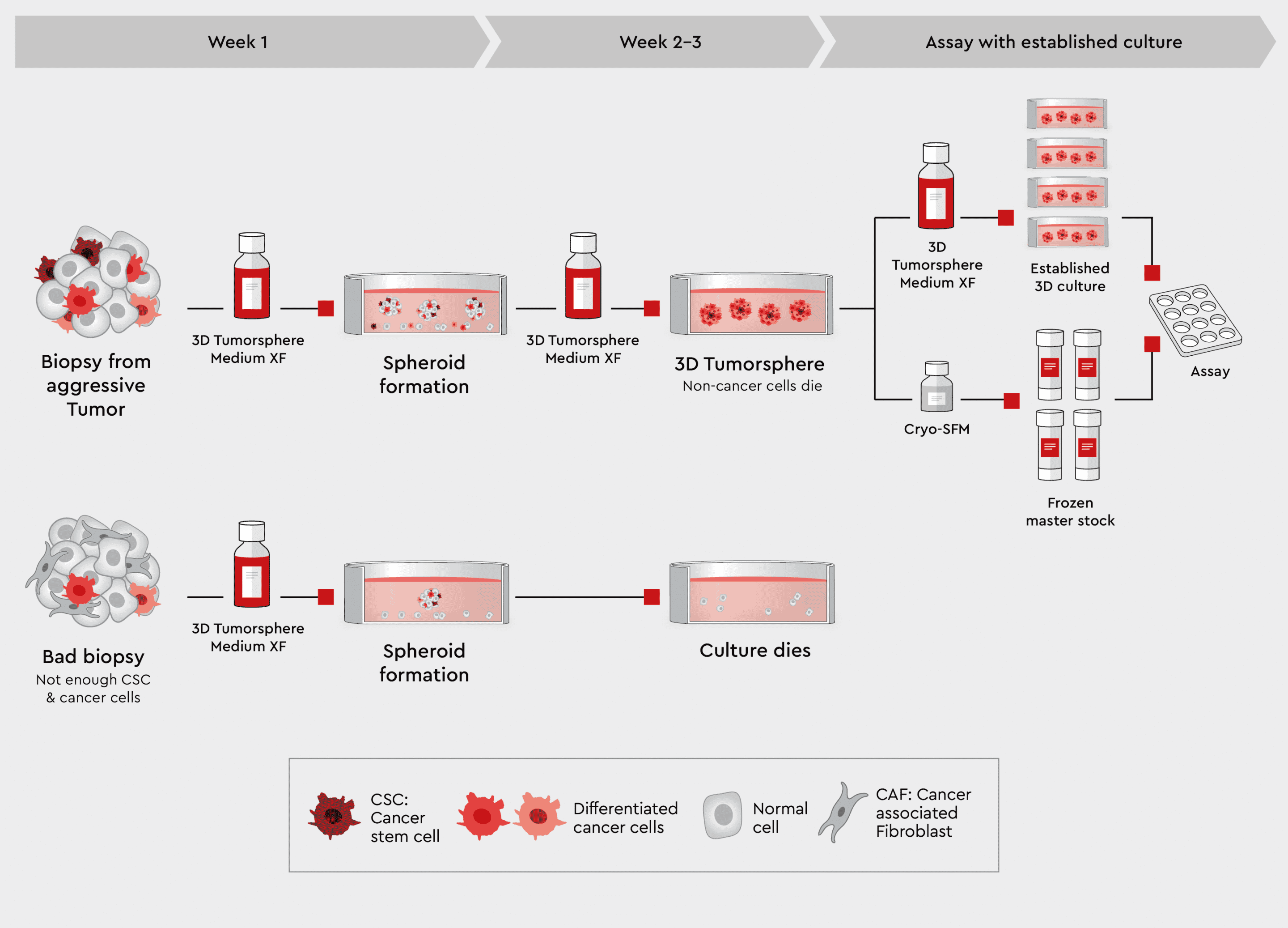
Figure 4: Workflow from biopsy to tumorspheres using the 3D Tumorsphere Medium XF. Using our 3D Tumorsphere medium XF, you can directly implement tumorspheres from aggressive tumor biopsies containing highly malignant cancer cells. Only cancer stem cells form new tumorspheres during passaging, sorting out non-cancer cells and conserving patient-specific tumor characteristics.
Biopsies also contain non-cancer cell types from the surrounding stromal cells or as an artefact of the biopsy procedure. These non-cancer cells are usually not desired in the culture. Since the 3D Tumorsphere Medium XF does not support non-cancer cells by design, those cells are eliminated in the culture. Rather than going through a selection procedure, cells from dissected biopsy samples can be added to the medium, sustaining the cancer cells but eliminating all other cells. The 3D Tumorsphere Medium XF medium is a synthetic medium which contains all components to support a cell culture continuously:
- Easy and direct suspension in 3D Tumorsphere Medium XF – no pre-selection for CSCs or highly malignant cancer cells needed
- Defined tumorspheres mimicking the original tumor biology
- No tumor/tissue-specific medium is needed since the 3D Tumorsphere Medium XF can be used with many tumor types
- Compatible with other media of our toolbox such as the Primary Cancer Cell Culture System
- The synthetic formulation allows user customization by adding other components to the medium
- Long-term storage of spheroids in our Cryo-SFM freezing medium
Experience has shown that no suitable 3D tumorspheres can be regularly obtained from the biological material. This has less to do with the experimental conditions or choice of medium but with the specific cancer cells in this sample. Either the sample did not contain CSCs making it impossible to develop tumor models, or the tumor’s malignancy was low. Usually, such biopsy should be discarded, as it will unlikely create material for proper assays. In case of a cancer type known to be of low malignancy, we recommend pre-selecting the pure CSCs in the Primary Cancer Culture System (PCCS).
Complex organoid models
How to build complex and robust cancer organoid models with the toolbox
As described in the previous sections, developing 3D Tumorsphere Models from biopsies and cancer cell lines aims to establish a model for screening and assay panels to identify and prioritize drug candidates. This section introduces our cancer toolbox for developing more complex 3D tumor models and organoids. More complex tumor models require the presence of non-cancer cells such as immune cells, vascular endothelial cells, and ECM components. The goal is to mimic the tumor microenvironment and tumor architecture.
To allow for co-cultivation of different cell types, our toolbox contains media that support cancer and non-cancer cells together, i.e., the Cancer Cell Line Medium XF (CCLM XF). One possible organoid design is described in our application note Cancer Organoid Model and shown in the figure below.
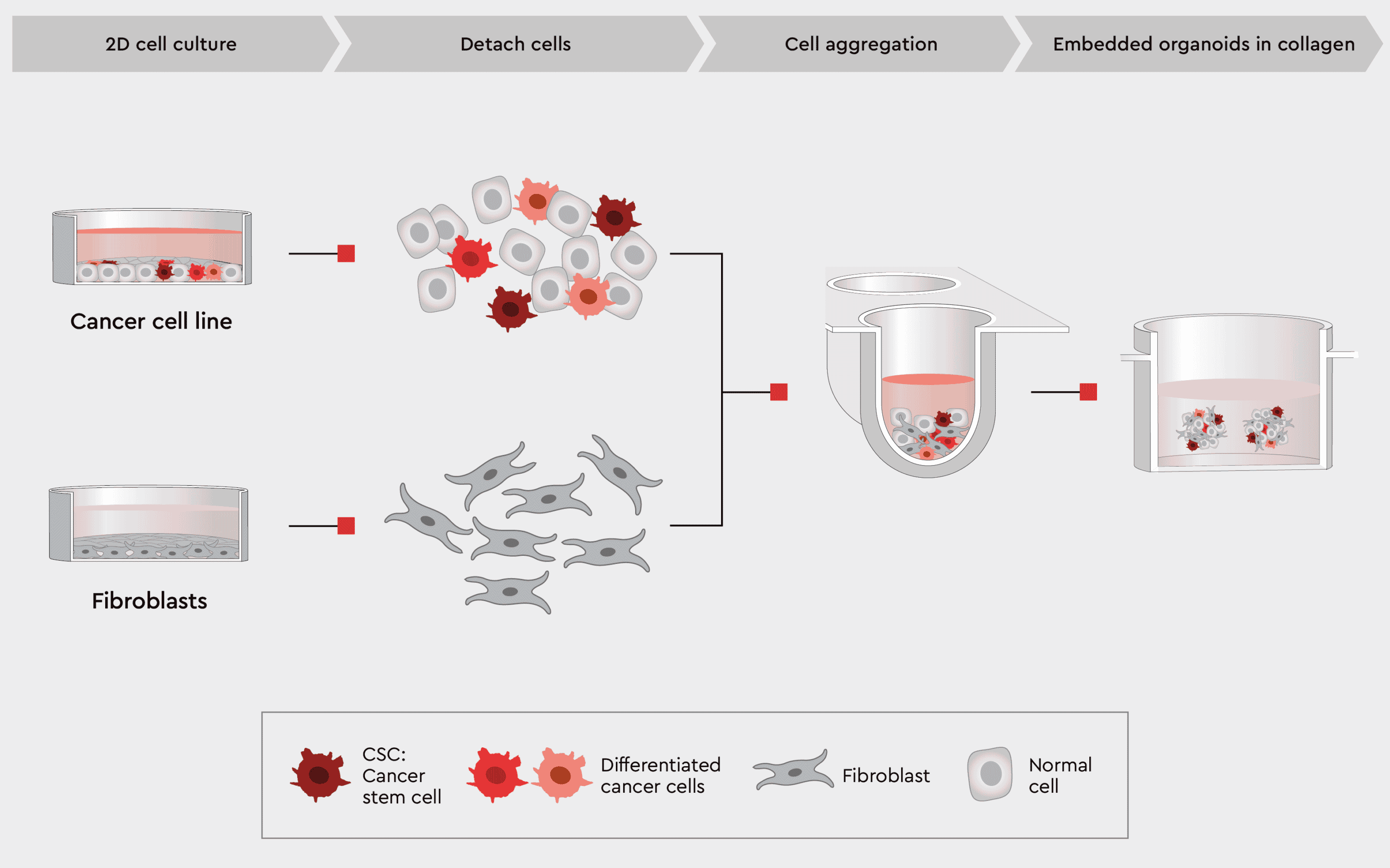
Figure 5: Workflow example to construct 3D organoids with cancer and non-cancer cell types.
The CCLM, by design, supports CSCs and other cancer cells but is also less stringent to other cell types like fibroblasts or immune cells.
Key advantages of our organoid model:
- The Cancer Cell Line Medium XF allow and support the presence of non-cancer cell types together in one culture system
- The media of our cancer toolbox are compatible with each other and can be used in the same workflow
- All media, including the Cancer Cell Line Medium XF, support healthy CSCs as the critical driver of tumor formation and metastasis
- The media can be combined with attachment matrices, i.e. collagen or the NCCD Reagent, to build an ECM
- The media are compatible with certain other ECM to mimic overall architecture, bioprinting and tumor stiffness
Below you can find exemplary result of the workflow using our Cancer Cell Line Medium XF to generate organoids displaying tumor characteristics.
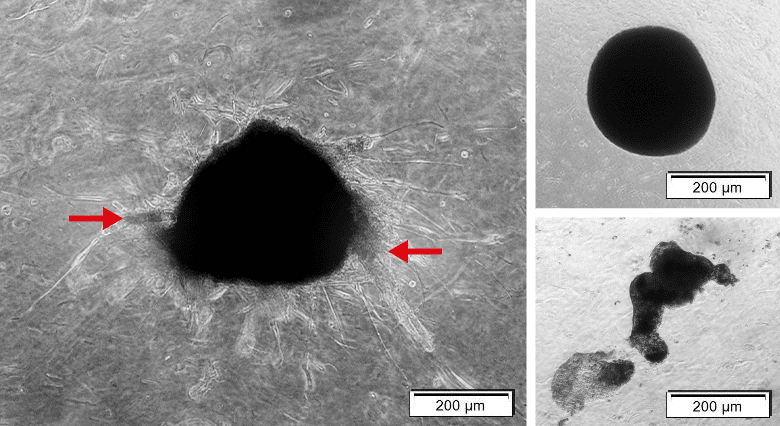
Figure 6: Complex organoid with tumor characteristics versus spheres. Left: Organoids containing MCF-7 and NHDF cells. Organoid shows sprouting (red arrow). Right upper: Organoid containing NHDFs solely is tickly packed with precise edges, but sprouting is only minor. Right lower: Organoid containing only MCF-7s showing only loose aggregation and no sprouting.
Do you want to learn how to use the media in such a model? Get in touch with our Experts.
Verification of tumor model
Verify your tumor models with the tumorsphere formation efficiency test
The most common method for quantifying the CSC population within a cancer culture is to calculate tumorsphere forming potential. The tumorsphere formation efficiency (TFE assay) estimates the number of tumorspheres formed by the number of single cells seeded. Since forming spheres from single cells is a property only attributed to stemlike cells, the TFE assay is a valuable qualitative and quantitative tool to measure the amount of cancer stem cells within a tumor or a cancer cell line. It correlates with cancer metastasis potential and aggressiveness.
Our 3D Tumorsphere Medium XF enables reliable single-cell derived sphere formation and serial passaging of tumorspheres due to the support of the CSCs. Thereby, sphere-forming CSCs can be gradually enriched when switching from a standard culture to a CSC-selective environment, resulting in a high cell proliferation rate.
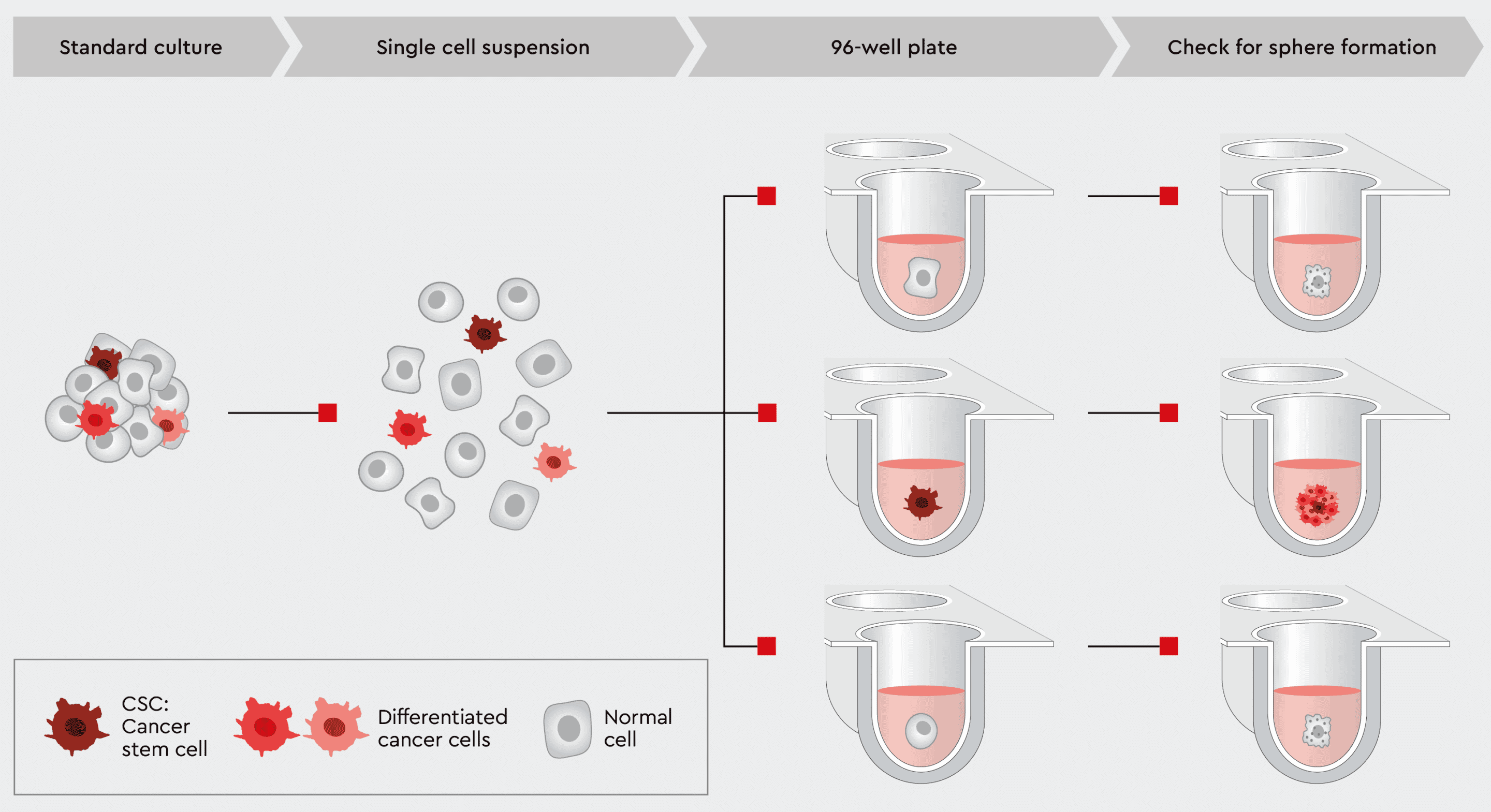
Figure 7: Tumorsphere formation efficiency (TFE) workflow using our 3D Tumorsphere Medium XF. Single cells are plated in a 96-well plate with one cell per well. After an adequate incubation time, cancer stem cells are capable of forming a new tumorsphere, while normal cells or further differentiated cancer cells undergo anoikis.
Using the TFE assay not only proves that your culture can form tumorspheres but is an excellent method to assess the tumorgenicity of your culture or to create an in vitro model for metastasis. Only through the presence of CSCs can your tumor model be passaged and expanded. Therefore, the better your culture conditions support CSCs, the more reliably new tumorspheres will form, and the proliferation rate will increase.
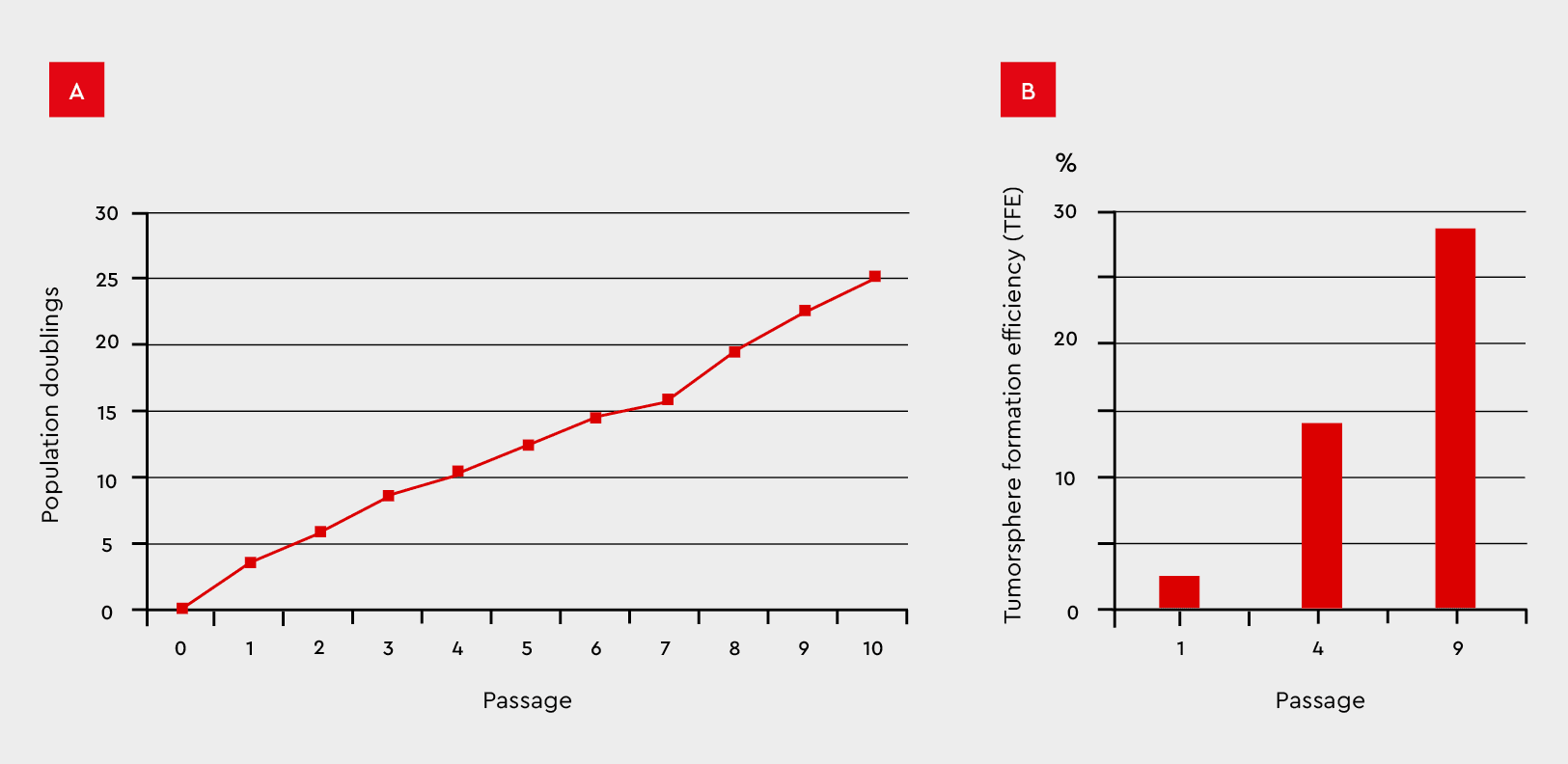
Figure 8: Tumorsphere formation efficiency during serial passages in the 3D Tumorsphere Medium XF. MCF-7 cancer cells were subjected to serial passaging by enzymatic dissociation. (A) Thereby, the tumorsphere formation and cell proliferation maintain over ten passages and (B) significantly increase TFE from 2% to 28%.
Discover our Application Note Determination of the Tumorsphere Formation Efficiency (TFE)




